Demyanov rearrangement
The Demyanov rearrangement (also known as the Demyanov reaction) is a name reaction in organic chemistry that was named after the Russian chemist Nikolai Jakowlewitsch Demjanow (1861–1938). This is a substitution reaction in which an amino group is replaced by a hydroxyl group .
Overview reaction
The reaction can be carried out with primary or secondary cyclic amines , it being possible for the products to be reduced or enlarged cyclic compounds . These are reacted with nitrous acid and water . When using secondary, cyclic amines, either simple substitution (top left) or a reduction of the ring to the primary alcohol (bottom left) takes place.
If a primary cyclic amine is used, the amino group can also be substituted by a hydroxyl group (top right). If the ring is enlarged, a secondary alcohol (bottom right) is obtained as a product.
Reaction mechanism
In all of the reactions mentioned, the nitrous acid is protonated by water . Under elimination of a water molecule from the resultant oxonium ion a formed nitrosonium which two resonance structures has.
The reactions of the nitrosonium ion with the starting materials mentioned are shown below. First of all, the basic mechanism is described, the cyclic structure being retained in the same form, in order to then explain the change in the cyclic structures.
Ring reduction
The ring reduction (left part of the overview reaction) should first be explained. The secondary, cyclic amine 1 is reacted with the nitrosonium ion, creating the cation 2 . The diazonium cation 4 is formed by intramolecular protonation of the hydroxyl group formed in cation 3 and with the elimination of water .
The subsequent elimination of a nitrogen molecule results in the formation of the carbenium ion 5 . The secondary alcohol 6 is obtained by subsequent hydrolysis . The number of ring-forming carbon atoms has been retained. The ring reduction follows the identical mechanism up to the formation of the carbenium ion 5 . However, this is not treated with water, but is subject to a 1,2-hydrogen shift. A new CC bond is thereby formed.
The intermediate is the primary carbenium ion 7 . The ring has been reduced in size from cyclopentane to cyclobutane . Subsequent work-up with water produces the primary alcohol 8 .
Ring enlargement
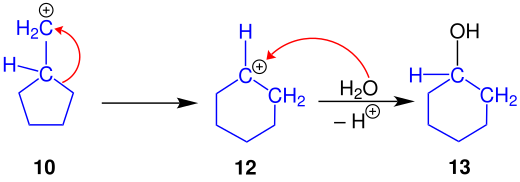
The conversion of a primary amine 9 follows the same mechanism up to the formation of the carbenium ion, which has already been described for the ring reduction. Therefore it will not be executed again. The resulting carbenium ion 10 differs only slightly from the carbenium ion 7 . Here, too, the carbenium ion 10 is worked up with water in order to arrive at the primary alcohol 11 . The five-membered, cyclic structure remains the same.
The ring expansion also starts from the primary carbenium ion 10 . This rearranges to the secondary carbenium ion 12 for reasons of stability .
Working up with water gives the secondary alcohol 13 .
Practical meaning
The Demjanov rearrangement is a method to expand or shrink four, five or six rings by one carbon atom. The products obtained can be used in a number of other reactions.
Problems
In both reactions it is difficult to isolate the resulting products, which has negative effects on the yield. The regioselectivity of the reaction is also problematic.
Similar rearrangement reactions
literature
- Z. Wang: Comprehensive Organic Name Reactions and Reagents , Vol 1. John Wiley & Sons, Hoboken, New Jersey 2009 , pp. 868-870, ISBN 978-0-471-70450-8 .
- Jie Jack Li: Name reactions, a collection of detailed reaction mechanism . Vol 1.Springer 2002 , p. 95. ISBN 3-540-43024-5 .
Individual evidence
- ↑ J. Diamond, WF Bruce, FT Tyson: Hexahydro-1-methyl-4-phenyl-4-acetoxyazepine and the Demjanov Rearrangement of 1-Methyl-4-phenylpiperidine-4-methylamine * , The Journal of Organic Chemistry 1965 , 30 (6) , 1840-1844, doi: 10.1021 / jo01017a030 .
- ↑ R.Kotani: Demjanov Rearrangement of 1-Methylcyclohexanemethylamine , The Journal of Organic Chemistry 1965 , 30 (2) , 350-354, doi: 10.1021 / jo01013a009 .
- ↑ AG Stern, A. Nickon Synthesis of brexan-2-one and ring-expanded congeners , Journal of Organic Chemistry 1992 , 57 (20) , 5342-5352, doi: 10.1021 / jo00046a015 .
- ↑ JB Jones, P. Price: Steroids and steroidases — XIX: Comparison of diazomethane and tiffeneau-demjanov homologations of 5α-3-oxosteroids. Evidence for predominant equatorial approach of the C-3 carbonyl group by diazomethane , Tetrahedron 1973 , 29 (14) , 1941-1947, doi: 10.1016 / 0040-4020 (73) 80128-0 .
- ↑ M. Nakazaki, K. Naemura, M. Hashimoto: Unusual consecutive rearrangements in the Demjanov ring-expansion reaction of 2- (aminomethyl) -D2d-dinoradamantane and 9- (aminomethyl) noradamantane , The Journal of Organic Chemistry 1985 , 48 ( 13) , 2289-2291, doi: 10.1021 / jo00161a033 .