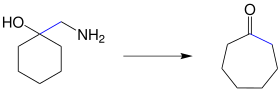
Tiffeneau ring extension
The Tiffeneau ring expansion (also Tiffeneau-Demjanow reaction , rearrangement or ring expansion ) is a name reaction from the field of organic chemistry and describes the synthesis of ring-expanded, cyclic ketones starting from 1-aminomethylcycloalcohols ( β-amino alcohol ).
Overview reaction
The reaction was discovered in two sub-steps. In 1901, the Russian chemist Nikolai Jakowlewitsch Demjanow (Russian: Николай Яковлевич Демьянов; 1861–1938) observed that the diazotization of aminomethylcycloalkanes produced cyclic ketones with ring expansion. This reaction was named after him as the Demyanov rearrangement . In 1937 Marc Tiffeneau (1873–1945), Weill and Tchoubar published their results on the diazotization of 1-aminomethylcyclohexanol, whereby cycloheptanone is formed:
The Tiffeneau-Demjanow reaction belongs to the group of rearrangements proceeding via carbenium ions , as does the Wagner-Meerwein rearrangement . There is a particular similarity to the pinacol rearrangement because of the 1,2-shift of the alkyl radical .
Reaction mechanism
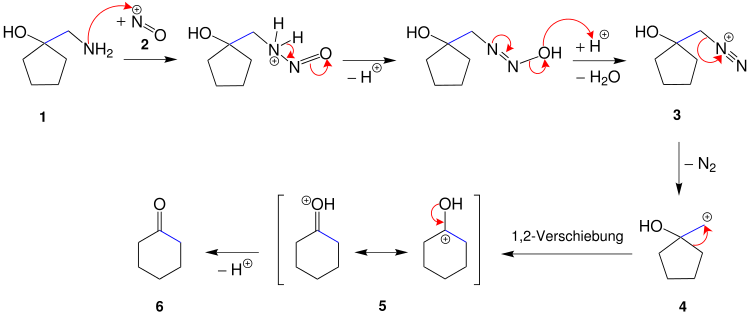
The reaction mechanism is to the example of cyclohexanone from 1-Aminomethylcyclopentanol 1 and dilute nitrous acid are shown. For this purpose, the first step is to produce the electrophilic nitrosyl cation 2 , which is attacked by the lone pair of electrons in the nitrogen in the amino group. When the diazotization is complete, a diazonium cation 3 is present.
Since aliphatic diazonium salts are not resonance-stabilized and therefore do not represent stable compounds, they tend to decompose and quickly split off a nitrogen molecule, creating a primary carbenium ion 4 . Primary carbenium ions are very unstable and rearrange immediately with a 1,2-shift of the alkyl radical, which leads to ring expansion and the formation of a mesomerism-stabilized carbenium ion 5 . The elimination of nitrogen and the rearrangement take place in concert, so the mechanism is formally not S N 1, but S N i. The subsequent deprotonation leads to cyclohexanone ( 6 ).
application
The reaction can be used to add a methylene group to cyclic ketones. For this purpose, nitromethane or hydrogen cyanide, for example , is added to the carbonyl group of ketone 1 and the corresponding reaction products (nitromethyl alcohol 2 or cyanohydrin 3 ) are hydrogenated to form β-amino alcohol 4 .
The rearrangement of the β-amino alcohol by the Tiffeneau-Demjanow reaction then gives the ring-expanded ketone 5 .
Individual evidence
- ↑ L. Kürti, B. Czakó: Strategic Applications of Named Reactions in Organic Synthesis - Background and Detailed Mechanisms . Elsevier Inc., 2005, ISBN 978-0-12-369483-6 , pp. 134-135 .
- ↑ shp-asso.org
- ↑ Marc Tiffeneau, Paul Weill, Bianca Tchoubar: Isomérisation de l'oxyde de méthylène cyclohexane en hexahydrobenzaldéhyde et désamination de l'aminoalcool correspondant en cycloheptanone . In: Comptes Rendus . 205, 1937, pp. 54-56.
- ↑ W. Uhl, A. Kyriatsoulis: Name and Keyword reactions in organic chemistry . Vieweg & Sohn, Braunschweig 1984, ISBN 3-528-03581-1 , pp. 150-151 .
- ↑ L. Kürti, B. Czakó: Strategic Applications of Named Reactions in Organic Synthesis - Background and Detailed Mechanisms . Elsevier Inc., 2005, ISBN 978-0-12-369483-6 , pp. 134-135 .
- ↑ L. Kürti, B. Czakó: Strategic Applications of Named Reactions in Organic Synthesis - Background and Detailed Mechanisms . Elsevier Inc., 2005, ISBN 978-0-12-369483-6 , pp. 134-135 .
- ↑ R. Brückner: reaction mechanisms: organic reactions, stereochemistry, modern synthesis methods . 3. Edition. Elsevier, Munich 2004, ISBN 3-8274-1579-9 , pp. 605-607 .
- ↑ diazotization
- ^ Francis A. Carey, Richard J. Sundberg: Organic Chemistry , 2. Corr. Reprint, Wiley-VCH-Verlag, 2004. ISBN 3-527-29217-9 .
- ↑ C. Ferri, G. Düsing, W. Lürken: Reactions of organic chemistry . Thieme, Stuttgart 1978, ISBN 3-13-487401-6 , p. 422 .