Rectification column

The rectification column is a process engineering device for the thermal separation of mixtures . Even if distillation columns are mostly used in common parlance , the principle of rectification is used in the majority of industrial apparatus, which has consequences for construction and operation. For the difference and the thermodynamic principles of distillation and rectification , reference is made to the corresponding article.
In chromatography , the comparable device is called a separation column .
Basic structure
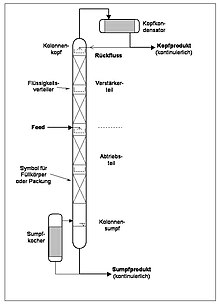
Although mostly only a "column" is spoken of, a useful rectification column also includes a number of other components, whereby it is initially irrelevant whether it is a rectification or a distillation column (see also the schemes below):
Column body
The actual column is a cylindrical tube, insulated to avoid heat loss, which in industry mostly consists of steel or higher alloyed stainless steels, while in the laboratory glass is mostly used. Due to its limited thermal resistance, plastic is only used in rare exceptions. The height of the column body is mainly dictated by the required quality of separation; the diameter through the mass flow of the mixture to be separated. Due to the usually great height, columns are divided into segments called “sections”.
Evaporator
In order to evaporate the mixture, there is an evaporator , also called a sump boiler, at the lower end of the column (in the sump) . This heat exchanger can be designed differently depending on the properties of the mixture and other boundary conditions: As a tube bundle integrated directly into the sump , double jacket, external natural circulation evaporator (as shown in the diagrams below) or forced circulation evaporator . Almost all conceivable heat sources can be used as the operating medium. In most cases it will be steam, but it could just as easily be hot water, smoke, electricity, or microwaves.
capacitor
A condenser is required to liquefy the mixture vapor at the top of the column (at the top) . Like the evaporator, this heat exchanger can also be integrated directly into the head or designed as an external device next to the column. It can also be operated with a large number of possible operating media such as water, cooling brine, refrigerant or air.
Internals
To intensify the heat and mass exchange between the gas and liquid phases , the column body is provided with internals which are selected on the basis of the properties of the mixture (e.g. high foaming) and operating mode of the column (e.g. vacuum). Since they have a decisive influence on the hydraulics and thus directly on the separation behavior of the column, they are also used to characterize the column type.
There are three types:
- In tray columns sieve, bubble-cap or valve trays are installed, at which the liquid is. The vapor is bubbled into the liquid through special slots or holes, creating a bubbly layer. A new temperature-dependent equilibrium between the liquid and gas phases is established on each of these floors. Ideally, one tray corresponds to a theoretical separation stage.
- Packed columns can be filled with different packings that cause good distribution of the liquid and turbulence in the gas flow. By enlarging the surface of the 5 to 80 mm large bodies, heat and material exchange are intensified and the separation capacity of the column is increased. Well-known examples are the Raschig ring (a hollow cylinder), Pall ring, Hiflow ring, Intalox saddle, Berl saddle and hedgehog. The random packings can be introduced into the column in an orderly, but mostly random manner (as a bed). Glass, ceramics, metal and plastics can be used as materials.
- Structured packings are a further development of the random packing and have a regularly shaped structure. In the case of packings, this makes it possible to reduce constrictions for the gas flow (with a considerable influence on the pressure loss). There are different designs of packs e.g. B. tissue or sheet metal packaging. Metal, plastic, glass and ceramic (depending on wettability) can be used as material. There are packs from many major manufacturers with different names and preferred areas of application.
For the reliable and effective operation of all column types, a uniform distribution of the gas and liquid phases over the entire cross section of the column is necessary. In the case of tray columns, this is already guaranteed due to the design, but with structured packings and especially packed columns, additional measures are required. After passing a certain packing height, the entire liquid phase is collected and redistributed over the cross section. The gas flow is also automatically equalized again. If this does not happen, the liquid tends to flow down the inner walls of the column, so that only a very limited contact is possible with the gas phase in the middle, which considerably reduces the separation efficiency.
Modes of operation
Discontinuous operation
Batch operation is mainly used to separate a multicomponent mixture into its various individual components with a single column - to fractionate it. Here, the mixture is placed in the bottom of the column and evaporated in the evaporator. At first, the component with the lowest boiling point preferably goes over into the gas phase and rises in the column body. Constant condensation and re-evaporation take place on the internals, which leads to an enrichment of the lowest-boiling component in the gas phase and the components with the higher boiling points in the liquid phase.
In the case of a distillation, the entire mixture liquefied at the top in the condenser is drawn off and collected as a fraction until a rise in the top temperature indicates the next component. In most cases a so-called intermediate fraction is then formed, which consists of a mixture of two components and is treated separately before the next component is finally collected. Theoretically, it is thus possible to obtain all individual substances one after the other in pure form until the entire original mixture has evaporated. In practice, however, from a certain minimum fill level, either fresh mixture is topped up or the rest is removed from the sump for further treatment.
The procedure for rectification is basically the same, but with the crucial difference that only a small part of the top condensate is drawn off and collected, while the majority is fed back into the column top as what is known as reflux. In this way, the separating effect is improved quite considerably, and the individual components can be of a much higher purity.
Continuous operation
While the laboratory mostly works in batches, most of the industrial columns are operated continuously. The mixture to be separated is fed in a constant flow at a fixed point in the column (feed), from where it trickles down over the internals into the bottom. During the intensive heat and mass transfer with the vapors rising from below, the lower-boiling components are "stripped out" of the liquid and rise again in the gas phase. The higher-boiling components continue to flow downwards until they are finally drawn off continuously from the sump, heavily enriched. The area between the bottom and the mixture feed is also referred to as the “stripping section” of the column.
Above the feed, the liquid trickling down from above causes the remaining higher-boiling components to be “washed out” from the gas phase into the liquid phase, with which they are transported into the sump. The heavily enriched, low-boiling components are liquefied at the head and partly fed back into the head as reflux, partly withdrawn in a steady stream. This refeeding is indispensable in continuous operation in order to ensure the effectiveness of the rectification section between the feed and the head.
In contrast to a batch, in a continuous column a multicomponent mixture can only be separated into, at best, a pure substance (low or low end boilers) and a mixture of the other components, which must be treated in further columns. But it is much easier to handle and automate.
Special columns
In addition to the variants described above, there are now a number of further developments. B. cause higher energy efficiency or lower investment costs, but are less flexible and more difficult to drive due to the greater specialization.
Dividing wall column
In this column, a vertical dividing wall runs over part of the height of the column, dividing the cross section into two sections. The liquid phase is collected above the dividing wall and distributed over the two column cross-sections in a selectable ratio. With correct design and operation, it is theoretically possible to separate a three-component mixture into its three pure components in a single column, which would normally require two conventional columns. By saving a complete column and peripherals, the significantly higher investment costs for a dividing wall column are more than compensated for. In addition, due to thermodynamic effects, the energy requirement is also lower than would be required for the system of two columns. However, the design, operation and control of such a column pose a challenge to process engineering.
Reactive rectification column
This column is a combination of a chemical reactor and a rectification column. In it, a reaction takes place on special packings, usually coated with a catalyst, while the reaction products are separated at the same time. In this way it is possible, for example, to obtain complete conversion of the raw materials in a single apparatus for a reaction which otherwise only takes place up to a certain equilibrium. Here, too, however, the design and operation of such a column is associated with a significantly higher level of effort than is the case with a simple column.
Columns with side draw
In the case of some mixtures, it may be necessary to remove a substream from the column at one or more points along the height of the column in order to avoid excessive enrichment. However, it is not possible to obtain a pure component from these side draws, but always only a mixture with a certain content of low and high boilers.
See also
literature
- Barbara Elvers, Giuseppe Bellussi (Ed.): Ullmann's Encyclopedia of Industrial Chemistry , 7th edition, Wiley-VCH, Weinheim 2011, ISBN 978-3-527-32943-4 .
- Werner Hemming, Walter Wagner: Verfahrenstechnik , 8th edition, Vogel, Würzburg 2008, ISBN 978-3-8343-3113-7
Web links
- Montz company - manufacturer of dividing wall columns, random packings, packings and general internals
- Raschig company - manufacturer of fillers and packs
- United random packing factories - manufacturer of packing cores and packings
- Sulzer Chemtech - manufacturer of random packings, trays of all kinds, packings, internals and dividing wall columns
- RVT Process Equipment GmbH - manufacturer of random packings, trays of all kinds, packings, internals