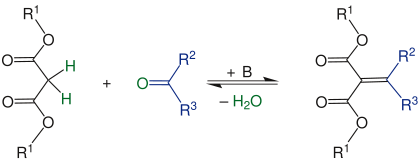
Knoevenagel reaction
The Knoevenagel reaction or Knoevenagel condensation is an important reaction in organic chemistry . It is a special case of the aldol reaction , in which ketones (R 2 = organyl group, R 3 = organyl group) or aldehydes (R 2 = H, R 3 = H or organyl group ) with particularly CH-acidic compounds (R 1 = organyl group), e.g. . B. malonic acid ester , acetoacetic ester or nitromethane are implemented. This creates unsaturated condensation products.
A special way of carrying out the Knoevenagel reaction is its Doebner variant. In it, the aldehyde / ketone is reacted with free malonic acid in pyridine and in the presence of an amine as a catalyst (often pyrrolidine or piperidine ). The resulting unsaturated dicarboxylic acid decarboxylates. In this way z. B. cinnamic acids very easily accessible.
The reaction was named after its discoverer Emil Knoevenagel and the Doebner variant after Oskar Doebner .
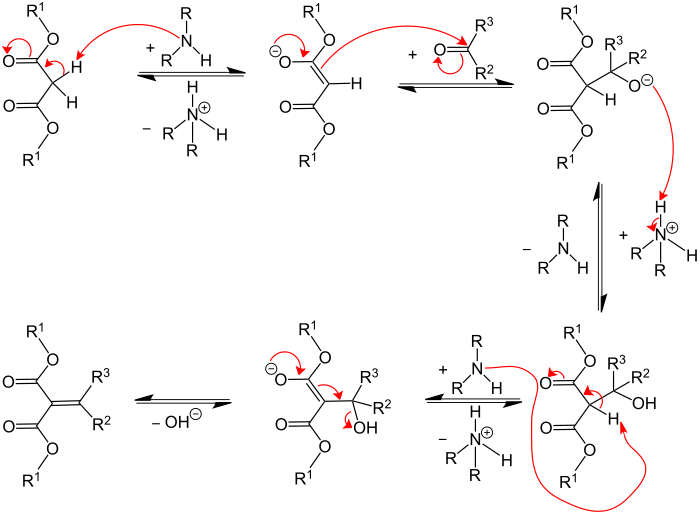
Reaction mechanism
In the first step, the CH-acidic compound (R 1 = organyl group ) is deprotonated by the base added as a catalyst, here a secondary amine. The resulting anion acts as a carbanion and nucleophilically attacks the carbonyl carbon of the carbonyl compound, while the oxygen of the carbonyl group is protonated. The result is an alcohol that can also be isolated as an intermediate. The unsaturated end product is finally formed with the elimination of water.
The Doebner variant offers easy access to α, β-unsaturated carboxylic acids, e.g. B. cinnamic acids (R = various substituents in different positions, e.g. -OCH 3 ):
In the above reaction equation, the radical R on the phenyl ring can be in the ortho , meta or para position (see substitution pattern ).
Weiss-Cook reaction
The Weiss-Cook reaction, also Weiss-Cook condensation or Weiss reaction, describes the reaction of an α-dicarbonyl (1,2-diketone) with two equivalents of 3-oxoglutarate under slightly acidic conditions (pH = 5) to form a cis -Bicyclo [3.3.0] octane-3,7-dione derivative, which proceeds according to the same reaction mechanism as in the Knoevenagel reaction. The reaction undergoes acid hydrolysis with subsequent decarboxylation.
During the cyclization , a number of thermodynamic equilibrium reactions are carried out , favoring the cis isomer. It has been observed that the Weiss-Cook reaction also proceeds under basic conditions. The reaction is used to produce fused compounds (e.g. polyquinanes and polyquinenes ).
Known products
literature
- Organikum , 16th edition, VEB Deutscher Verlag der Wissenschaften, Berlin 1985, ISBN 3-326-00076-6 , pp. 458–459.
- Peter Sykes : reaction mechanisms - an introduction , 8th edition, VCH, Weinheim 1982, ISBN 3-527-21090-3 , p. 258.
Individual evidence
- ^ Emil Knoevenagel: In Condensation of Malonic Acid with Aromatic Aldehydes by Ammonia and Amines , Reports of the German Chemical Society 1898 , 31 , 2596–2619, doi : 10.1002 / cber.18980310308 .
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents , Wiley Verlag, 2009, pp. 1621-1925, ISBN 978-0-471-70450-8 .
- ↑ Oskar Doebner: About the unsaturated acids homologous to sorbic acid with two double bonds , reports of the German chemical society , 1902 , 35 , pp. 1136–1147, doi: 10.1002 / cber.190203501187 .
- ↑ Weiss-Cook Condensation . (Weiss Reaction, Weiss ‐ Cook Reaction). In: Wiley Online Library (Ed.): Comprehensive Organic Name Reactions and Reagents . October 15, 2010, doi : 10.1002 / 9780470638859.conrr662 (English).