Unicorn-Brunner reaction
The Einhorn-Brunner reaction is a name reaction in organic chemistry . It was named after the German chemist Alfred Einhorn , who published it in 1905. In 1914, the reaction was expanded by Karl Brunner (1855-1935) and therefore known as the Einhorn-Brunner reaction . With the help of the reaction, 1,2,4-triazoles can be synthesized from hydrazines and diacylamines.
Overview
In the presence of an acid, hydrazines react with diacylamines to form 1,2,4-triazoles:
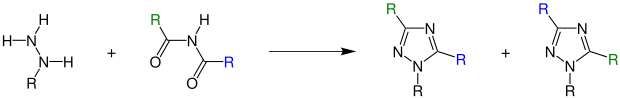 R = organic radical , the radicals can be the same but also different
R = organic radical , the radicals can be the same but also different
In the event that the organic radicals on the diacylamine are different, a mixture of isomeric triazoles is formed. The remainder of the imide, which is derived from the stronger acid, is found mainly in the 3-position of the triazole. If there are two identical acyl groups on the amine , only one product is formed.
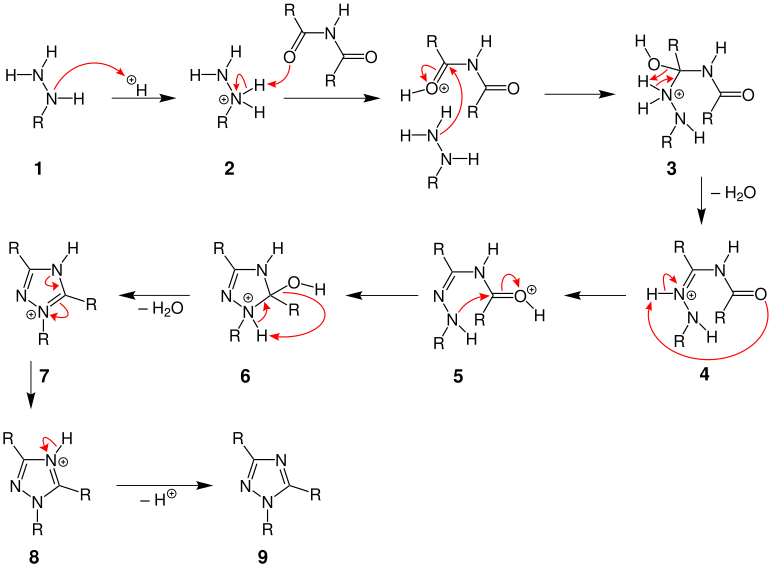
mechanism
For the sake of simplicity, only the reaction to one of the resulting triazole isomers is shown in the mechanism presented here .
First, the more electron-rich nitrogen atom in hydrazine derivative 1 is protonated , with cation 2 being formed. 2 transfers a proton to the oxygen atom of the carbonyl group of the diacylamine. In addition, the amino group attacks the more electrophilic carbonyl group, which ensures that an ammonium intermediate 3 is formed. The iminium ion 4 is formed from 3 by splitting off water . This is followed by a 1.5 proton shift from the nitrogen atom to the carbonyl group with the formation of the mesomeric-stabilized intermediate 5 (only a mesomeric boundary structure is shown here). The carbon atom of the protonated carbonyl group in 5 is now attacked intramolecularly by the nitrogen atom, which leads to the closure of a five-membered ring to form 6 . With elimination of water and a proton, the aromatic 9 , a 1,2,4-triazole, is formed via intermediate stages 7 and 8 .
See also
Individual evidence
- ↑ a b c d Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents. 3 volume set. John Wiley & Sons, Hoboken, NJ 2009, ISBN 978-0-471-70450-8 , pp. 971-972.
- ^ Karl Brunner: A new way of representing secondary acid amides . In: Reports of the German Chemical Society . tape 47 , no. 3 , July 1914, p. 2671-2680 , doi : 10.1002 / cber.19140470351 .
- ↑ Alfred Einhorn , Eduard Bischkopff, Bruno Szelinski, Gustav Schupp, Eduard Spröngerts, Carl Ladisch, Theodor Mauermayer: About the N-methylol compounds of the acid amides [first treatise.] In: Justus Liebigs Annalen der Chemie . tape 343 , no. 2–3 , 1905, pp. 207-305 , doi : 10.1002 / jlac.19053430207 .
- ↑ Karl Brunner: A new way of representing triazoles . In: Monthly books for chemistry and related parts of other sciences . tape 36 , no. 7–8 , July 1915, pp. 509-534 , doi : 10.1007 / BF01524682 .
- ^ MR Atkinson, JB Polya: Triazoles. Part II. N-substitution of some 1: 2: 4-triazoles . In: Journal of the Chemical Society . (Resumed), 1954, p. 141-145 , doi : 10.1039 / JR9540000141 .
- ↑ KT Potts: The Chemistry of 1,2,4-Triazoles. In: Chemical Reviews . tape 61 , no. 2 , April 1961, p. 87-127 , doi : 10.1021 / cr60210a001 .