Flash evaporation
Flash evaporation is the generation of steam when the pressure is reduced in a closed container filled with liquid and under overpressure (to the environment).
Classic examples are the pressure drop in the drum of a steam generator ( forced circulation boiler or natural circulation boiler ) or the bubbling when opening a mineral water bottle.
During the flash evaporation, the liquid in the drum is overheated and steam is generated . In the mineral water bottle, the pressure in the bottle is reduced by opening the lid, so that the carbon dioxide dissolved in the water "gasses". However, this is not about the transition of a liquid into the gaseous state of aggregation , but rather the escape of a dissolved gas from a liquid, which is expressed in a similar way (formation of gas bubbles, etc.).
Physical consideration
Flash evaporation occurs in a closed container that is filled with saturated boiling liquid and the associated vapor phase when the pressure is lowered by releasing an outlet cross-section (e.g. a valve).
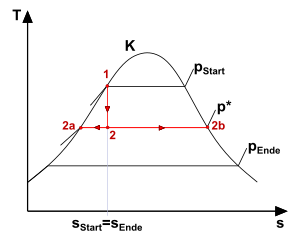
In the closed container, the liquid has a pressure p start at a given temperature T 1 - the saturation pressure of the corresponding temperature, which is determined by the boiling curve in state 1 in the TS diagram . If the valve is opened, the pressure drops, the new pressure level p * (p * <p start ) propagates in the container with wave propagation speed a . The temperature, on the other hand, cannot adapt at the same speed, since it is subject to heat and mass transfer mechanisms at the water / steam phase boundary , which are slower. This time delay can be referred to as the boiling delay time .
In this state the liquid is superheated, it and its vapor are no longer in thermodynamic equilibrium . This state means that the liquid has to give off heat - in this case, this happens through an energy input to boiling nuclei and existing vapor bubbles in the container. This leads to the growth of bubbles and their rise and ultimately to the bulging of the liquid and the storage of mass and energy in the form of vapor - the process commonly referred to as " boiling ". The steam thus transports energy out of the container.
Assuming isentropic processes, the mixture runs through the vertical isentropic "s" from the initial state T 1 (p start ) to the new final state p end . If one considers a snapshot of state 2, which is reached after opening the valve and lies between the initial and final state, the expansion evaporation proceeds in such a way that the liquid from point 2 to point 2a and thus the state "saturation boiling", the Steam reaches the state 2b, "saturated steam".
If the thermodynamic equilibrium between the phases is reached again, the flash evaporation comes to a standstill. The composition of the liquid and vapor phases for a mixture of chemical substances can be determined by a flash calculation .
The process of mass storage in the form of steam is used in steam generators with a drum, e.g. B. natural circulation or forced circulation boiler , since here after a pressure reduction to increase performance immediately - depending on the design and size - about 200 to 600 kg of steam per bar of pressure reduction are available. In power plants, at operating pressures of 70–200 bar, pressure reliefs of 5–10% of the nominal pressure are common when the load changes. This corresponds to a rate of change in performance of around 5–10% per minute.
In key words
- Lowering the pressure leads to overheating of the liquid
- New pressure spreads in the container with wave propagation speed a
- Temperature change is slowed down by heat and mass transfer at the phase boundaries
- Thermodynamic equilibrium no longer exists
- Overheating is reduced by transferring energy to boiling nuclei and existing vapor bubbles
- Energy input into boiling nuclei and bubbles leads to bubble growth and rise of the bubbles
- Storage of mass in the form of steam removes the temperature from the system
- New thermodynamic equilibrium is reached
literature
- SG Kandlikar: Handbook of Phase Change - Boiling and Condensation. Taylor and Francis publisher, 1999
- F. Mayinger: Flow and heat transfer in gas-liquid mixtures. Springer-Verlag, 1982
- K. Stephan: Heat transfer when condensing and boiling. 4th edition, Springer-Verlag, 1996