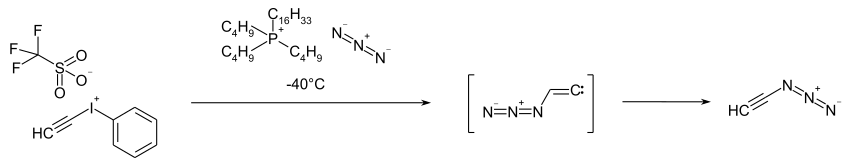
Ethynyl azide
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|

|
|||||||
| General | |||||||
| Surname | Ethynyl azide | ||||||
| other names |
|
||||||
| Molecular formula | C 2 HN 3 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 67.05 g mol −1 | ||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Ethinylazide ( azidoacetylene ) is an unstable organic compound that can be seen as both an organic azide and a monosubstituted ethyne . The nitrogen content in the molecule is 62.7%.
history
A first, unsuccessful attempt to establish the connection was published as early as 1910. Starting from the vinyl azide , 1-azido-1,2-dibromoethane was obtained by bromination . Its double dehydrohalogenation to give the target compound failed. In the years that followed, further unsuccessful attempts at synthesis were reported. The first production was achieved in 2012 by a group from the TU Chemnitz .
Presentation and extraction
It is produced by reacting ethynylphenyliodonium trifluoromethanesulfonate or tetrafluoroborate with hexadecyltributylphosphonium azide at −40 ° C.
properties
Ethinylazide is a highly explosive compound and can only be safely handled in solution. Characterization was carried out by means of 1 H, 13 C, 15 N NMR spectroscopy and IR spectroscopy . The half-life in solution in dichloromethane is about 17 hours at −30 ° C. One trapping reaction is the reaction with cyclooctyne, with a corresponding triazole derivative being formed. The decomposition of the compound proceeds via a carbene intermediate after nitrogen has been split off . The decomposition in the presence of 2,3-dimethylbut-2-ene gives a mixture of 1-cyano-2,2,3,3-tetramethylcyclopropane and 4,5-dimethylhex-4-enenitrile.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Martin Onslow Forster , Herbert Newman: The triazo-group. Part XV: Triazoethylene (vinylazoimide) and the triazoethyl halides. In: Journal of the Chemical Society, Transactions . 97, 1910, pp. 2570-2579, doi : 10.1039 / CT9109702570 .
- ↑ Boyer, JH; Mack, CH; Goebel, N .; Morgan, LR: Reactions of Sodium Phenylacetylide and Sodium Alkoxide with Tosyl and Mesyl Azides in J. Org. Chem. 23 (1958) 1051-1053, doi : 10.1021 / jo01101a604 .
- ↑ Boyer, JH; Selvarajan, R .: Photolysis of vic-triazoles in Tetrahedron Lett. 10 (1969) 47-50, doi : 10.1016 / S0040-4039 (01) 97649-X .
- ↑ Garibina, VA; Leonov, AA; Dogadina, AV; Ionin, BI; Petrov, AA: J. Gen. Chem. USSR (Engl. Trans.) 55 (1985) 1771-1781.
- ↑ a b c d e f g Banert, K .; Arnold, R .; Hagedorn, M .; Thoss, P .; Auer, AA: 1-Azido-1-Alkynes: Synthesis and Spectroscopic Characterization of Azidoacetylene in Angew. Chem. 124 (2012) 7633-7636, doi : 10.1002 / anie.201203626 .
- ↑ Porchov, E .; Auer, AA; Banert, K .: Ab Initio Study of Molecular Properties and Decomposition of 1-Azidoalkynes - A Challenge for Experimentalists in J. Phys. Chem. 111 (2007) 9945-9951, doi : 10.1021 / jp072566g .