Fluoroacetone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
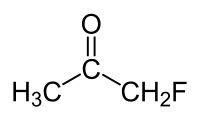
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Fluoroacetone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 5 FO | |||||||||||||||
| Brief description |
colorless clear to yellowish liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 76.1 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.054 g cm −3 |
|||||||||||||||
| boiling point |
75 ° C |
|||||||||||||||
| Refractive index |
1.37 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Fluoroacetone (also called monofluoroacetone ) is a fluorinated derivative of acetone with the empirical formula C 3 H 5 FO.
presentation
Fluoroacetone can be obtained by reacting triethylamine trihydrofluoride with bromoacetone .
properties
Fluoroacetone is a colorless, clear to pale yellow liquid at room temperature . It has a refractive index of 1.37 at 20 ° C and changes to a gaseous state from 75 ° C. Fluoroacetone is highly flammable, it has a flash point of 7 ° C. Above the flash point, the vapors of fluoroacetone can form an explosive mixture with air.
use
Fluoroacetone is or has not been used as an eye warfare agent , in contrast to other halogenated acetone derivatives such as bromoacetone or chloroacetone . It is a starting material for the production of higher fluoroketones.
Individual evidence
- ↑ a b c d e f Datasheet Fluoroacetone, 98% from Sigma-Aldrich , accessed on November 7, 2014 ( PDF ).
- ↑ Jamie L. Adcock, Ronald Eric Banks, Andreas Bulan, James Burdon, Karl Christe, Wojciech Dmowski, Georgii G. Furin, Udo Groß, Gerald B. Hammond, Mikhail Kremlev, Richard J. Lagow, Bernard Langlois, Dayal T. Meshri , Ralf Miethchen, R. Perry, Klaus Pohmer, Richard L. Powell, Michael Harold Rock, Shlomo Rozen, Stephan Rüdiger, Günter Siegemund, Jörn Stölting, LR Subramanian, Clarice Evelyn Mabel Tatlow, Tetsuro Tojo, Klaus Ulm, Nobuatsu Watanabe, Lev M. Yagupolskii, Yurii L. Yagupolskii, Marko Zupan: Organo-Fluorine Compounds - Fluorinating Agents and Their Application in Organic Synthesis (= Houben-Weyl Methods of Organic Chemistry . Vol. E 10a). 4th edition. Georg Thieme, 2014, ISBN 978-3-13-218004-8 , pp. 138 ( limited preview in Google Book search).
- ^ RD Chambers: Organofluorine Chemistry: Techniques and Synthons . Springer Science & Business Media, 1997, p. 165 ( limited preview in Google Book search).

