Fukuyama reduction
The Fukuyama reduction is a name reaction in organic chemistry from 1990. It was named after the Japanese chemist Tohru Fukuyama (* 1948) and is used for the synthesis of aldehydes . For this purpose a is thiol ester with triethylsilane in the presence of a palladium - catalyst implemented.
Overview reaction
The reaction takes place between a thiolester and triethylsilane. Finely divided palladium on activated carbon is used as a catalyst. The radical R in the structural formulas of the thiolester and aldehyde stands for an organic radical .
The Fukuyama reduction is used in the synthesis of aldehydes from carboxylic acids. In this synthesis route, the carboxylic acids act as starting materials from which thiolesters are synthesized for the Fukuyama reduction. In this way, a further reduction of the aldehyde to alcohol can be avoided. At the same time, the Fukuyama reduction is a particularly mild process that numerous functional groups survive unchanged.
Catalytic cycle
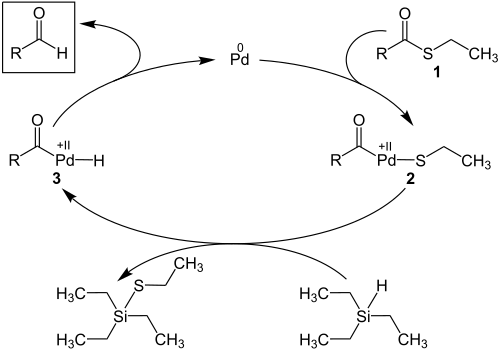
The Fukuyama reduction is catalyzed by palladium. This is a cross-coupling , which should be illustrated by the following catalytic cycle :
First, thiolester 1 reacts with the palladium catalyst to form intermediate 2 . Since the palladium is oxidized, this step is called oxidative addition. The reaction of intermediate 2 with the triethylsilane to intermediate 3 follows . Because organic residues are exchanged between two organometallic compounds, this process is a transmetalation . Finally, the palladium is eliminated from intermediate 3 in a reductive elimination (reversal of the oxidative addition) and an aldehyde is formed.
See also
- Glaser coupling
- Heck reaction
- Negishi clutch
- Sonogashira coupling
- Stephens-Castro coupling
- Silence coupling
- Suzuki clutch
Individual evidence
- ↑ Tohru Fukuyama, Shao Cheng Lin, Leping Li: Facile reduction of ethyl thiol esters to aldehydes: application to a total synthesis of (+) - neothramycin A methyl ether . In: Journal of the American Chemical Society . tape 112 , no. 19 , 1990, pp. 7050-7051 , doi : 10.1021 / ja00175a043 .
- ↑ a b Tohru Fukuyama; Hidetoshi Tokuyama: Palladium-Mediated Synthesis of Aldehydes and Ketones from Thiol Esters . In: Aldrichimica Acta . tape 37 , no. 3 , 2004, p. 87-96 ( sigmaaldrich.com [accessed June 30, 2017]).
- ↑ Oliver Reiser: Palladium-catalyzed carbon-carbon coupling reactions: synthetic methods . In: Chemistry in Our Time . tape 35 , no. 2 , 2001, p. 94-100 , doi : 10.1002 / 1521-3781 (200104) 35: 2 <94 :: AID-CIUZ94> 3.0.CO; 2-M .