Fumonisin B 1
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Fumonisin B 1 | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Fumonisin B 1 | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 34 H 59 NO 15 | ||||||||||||||||||
| Brief description |
light brown solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 721.83 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Fumonisin B 1 is a mycotoxin produced by various Fusarium species, including Fusarium verticillioides . It belongs to the group of fumonisins .
properties
Fumonisin B 1 occurs mainly in maize, wheat and other cereals and is an important food poison in agriculture . Fumonisin B 1 is hepatotoxic and nephrotoxic . Chronic poisoning leads to equine leukoencephalomalacia (ELEM) in horses and porcine pulmonary edema syndrome in pigs. Both of these diseases are characterized by disorders of the sphingolipid metabolism and cardiovascular dysfunction.
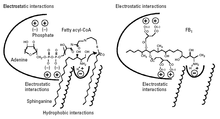
Fumonisin B 1 is biosynthesized via a polyketide pathway. It consists of two parts, one of which (amino alcohol) is adsorbed on the binding site for sphinganine or sphingosine and one (tricarballylic acid) is attached to the binding site for acyl-CoA in the ceramide synthase . As a result, the ceramide synthase is competitively inhibited . A synthetic derivative ( N -palmitoyl-AP1) with a palmitoyl group instead of the tricarballyic acid group inhibits ceramide synthase even more.
Individual evidence
- ↑ a b c data sheet fumonisin B 1 from Sigma-Aldrich , accessed on May 14, 2017 ( PDF ).
- ↑ European Commission: Opinion of the Scientific Committee on Food on Fusarium-toxins Part 3: Fumonisin B 1 (FB 1 ) (PDF; 185 kB).
- ↑ AH Merrill, MC Sullards, E. Wang, KA Voss, RT Riley: Sphingolipid metabolism: roles in signal transduction and disruption by fumonisins. In: Environmental health perspectives. Volume 109 Suppl 2, May 2001, pp. 283-289, PMID 11359697 , PMC 1240677 (free full text).