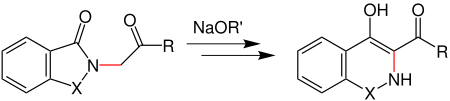
Gabriel-Colman rearrangement
The Gabriel-Colman rearrangement is a name reaction from organic chemistry. The rearrangement reaction was discovered in 1900 by Siegmund Gabriel and James Colman and named after them.
In the Gabriel-Colman rearrangement, an ester of phthalimide or saccharine is rearranged to a substituted isoquinoline with the help of an alcoholate . The functional group X can accordingly be either a carbonyl or sulfonyl group .
Reaction mechanism
The first reaction step is the nucleophilic addition of a strong base (e.g. a methanolate ) to one of the carbonyl carbon atoms of the phthalic acid derivative 1 . The ring opening creates the anion 2 , which quickly rearranges to form the carbanion 3 . The prerequisite for this is an enolizable hydrogen on the adjacent carbon atom. By cyclization of the carbanion and subsequent elimination of Methanolats the product produced 4 .
The isoquinoline derivative 4 shown has tautomers :
Individual evidence
- ↑ Gabriel, S .; Colman, J .: On the action of sodium alkylates on phthalylglycine ester and its homologues Chem. Ber. 1900 , 33 , pp. 980-995, DOI: 10.1002 / cber.190003301172 .
- ↑ Gabriel, S .; Colman, J .: About a rearrangement of the phthalimidoketones Chem. Ber. 1900 , 33 , pp. 2630-2634, DOI: 10.1002 / cber.190003302209 .
- ↑ Jie Jack Li: Name reactions, a collection of detailed reaction mechanism . 5th edition. Springer 2014, p. 275, ISBN 978-3-319-03979-4 .
- ↑ Hill, JHM: Mechanism of the Gabriel-Colman Rearrangement J. Org. Chem. 1965 , 30 , pp. 620-622, DOI: 10.1021 / jo01013a078 .