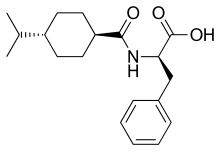
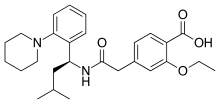
Glinide
Glinides are drugs from the group of oral antidiabetic agents that can be used in type 2 diabetes mellitus . In terms of their structure and their mechanism of action, they are closely related to sulfonylureas , like these, they stimulate the body's own insulin secretion and are therefore sometimes also referred to as sulfonylurea analogues. The currently available representatives of the group are nateglinide and repaglinide . In Germany, with effect from July 1, 2016, Glinide may only be prescribed at the expense of statutory health insurance in medically justified individual cases for patients with impaired kidney function.
Pharmacological properties
Pharmacodynamics
Like sulfonylureas, glinides work by blocking ATP- sensitive potassium channels on the β cells of the pancreas . As a result of this blockage, the resting membrane potential , which is essentially maintained by the diffusion of potassium ions, breaks down , and membrane depolarization occurs. This leads to the opening of voltage-dependent calcium channels and calcium ions flow into the cell interior. Insulin -containing vesicles fuse more intensely with the cell membrane ( exocytosis ), there is an increased release of insulin. Because of this mechanism of action, the glinides are among the insulinotropic antidiabetic agents.
Pharmacokinetics
Differences to the sulfonylureas can be found in the pharmacokinetic properties of the glinides. Particularly noteworthy are the rapid absorption and the short half-life. For example, with nateglinide and repaglinide, maximum plasma concentrations are reached within about one hour, with the frequently used sulfonylureas glibenclamide and glimepiride after about two to three hours. The short time of the flooding is also the reason why the glinides should be taken shortly before the main meals. The bioavailability of the glinides is 60 to 75%, slightly lower than that of the sulfonylureas with 90 to 100%. The metabolism of the glinids takes place through the cytochrome P450 enzyme system: nateglinide is v. a. by CYP2C9 , repaglinide v. a. degraded by CYP2C8. During the elimination, the glinides show an opposite behavior: the metabolites of nateglinide are mainly excreted renally (via the kidneys ), while metabolites of repaglinide are mainly excreted in the bile - ie via the bile and ultimately the stool .
Clinical information
Application areas (indications)
Glinides are used in type 2 diabetes mellitus. According to the practice guideline of the German Diabetes Society , they do not belong to the drugs of first choice, but can be used when adequate control of the metabolism through diet, weight reduction and physical activity and monotherapy with the biguanide metformin is not possible. In addition to the combination with metformin, repaglinide is also approved for monotherapy, nateglinide only for combination therapy with metformin.
Contraindications (contraindications)
As insulinotropic antidiabetic agents, the glinides are contraindicated in type 1 diabetes mellitus. In these patients, the β cells of the pancreas are destroyed by autoimmune reactions , and insulin release is no longer possible. Furthermore, Glinide must not be used in severe liver diseases, in diabetic ketoacidosis, or during pregnancy and breastfeeding. As with all drugs, it must not be used in patients with known hypersensitivity to drugs or auxiliary substances.
In the case of repaglinide, simultaneous use with gemfibrozil - a drug that has an effect on blood lipid levels - is also contraindicated. Gemfibrozil is a potent inhibitor of the cytochrome P450 isoenzyme CYP2C8, which plays a key role in the breakdown of repaglinide. If this enzyme is inhibited by taking gemfibrozil, the repaglinide plasma levels rise many times over, which is accompanied by an uncontrollable increase in the blood sugar-lowering effect.
Drug interactions
A variety of drug interactions can occur due to the mechanism of action and pharmacokinetics of glinides . For example, ACE inhibitors increase the blood sugar-lowering effect, while diuretics , corticosteroids or sympathomimetics lead to a reduced effect. As shown by the example of the contraindication of taking repaglinide and gemfibrozil at the same time, inhibitors and - analogous to inducers - the cytochrome P450 enzymes can have a considerable influence on the plasma level and thus the therapeutic effect of the glinide.
Adverse effects (side effects)
Since the release of insulin by insulinotropic antidiabetic drugs is independent of the current blood glucose level, there is a risk of hypoglycemic reactions (hypoglycaemia) when using glinides . Due to the short half-life of glinides, however, the risk of hypoglycaemia at night is said to be lower than with sulfonylureas. Other side effects often include gastrointestinal disorders such as abdominal pain, nausea, vomiting, diarrhea, and hypersensitivity reactions (allergic reactions), increased liver enzymes, and, with repaglinide, visual disturbances, which are equally rare with both glinides, often with nateglinide and rarely with repaglinide.
Expediency of Glinids
Glinide should no longer be reimbursed from October 2010 at the request of the Federal Joint Committee (G-BA) of the statutory health insurances (GKV) , because the benefits are not sufficiently proven by studies. The Federal Ministry of Health rejected this application to exclude the ordinance in March 2011 on the grounds that the inappropriateness and inefficiency of the Glinide had not been proven.
The G-BA sued the Federal Ministry of Health in this matter and won the case before the State Social Court of Berlin-Brandenburg with a judgment dated May 27, 2015, which is now legally binding. On February 18, 2016, the G-BA decided to put the regulation restriction into effect by publication in the Federal Gazette on July 1, 2016. From this day on, Glinide can only be prescribed in medically justified individual cases at the expense of the statutory health insurance.
Trade names
| Important note: Trade names and dosage forms of medicinal substances are not subject to any standardization. They can therefore differ in individual countries. |
The following drugs from the group of glinides are currently commercially available for therapy:
- Nateglinide : Starlix (A, D, CH), Starlix mite (CH), Trazec (A)
- Repaglinide : Repaglinide - Generika , NovoNorm (A, D, CH), Prandin (A)
- Mitiglinide : Glufast (J)
Individual evidence
- ↑ a b c Aktories K., Förstermann U., Hofmann F., Starke K .: General and Special Pharmacology and Toxicology. 9th edition. Urban & Fischer Verlag / Elsevier Verlag, Munich / Jena 2006, ISBN 978-3-437-44490-6 , pp. 626–629.
- ↑ G-BA puts regulation restriction for Glinide into force - Federal Joint Committee. In: www.g-ba.de. Retrieved June 6, 2016 .
- ↑ a b Mutschler E., Geisslinger G., Kroemer HK., Ruth P, Schäfer-Korting M .: drug effects. Textbook of pharmacology and toxicology. 9th edition. Scientific publishing company. Stuttgart 2008. p. 419. ISBN 3-8047-1952-X .
- ↑ German Diabetes Society: Diabetes mellitus type 2, DDG practice guideline (PDF; 856 kB), accessed on January 23, 2013.
- ↑ Richard Daikeler, idols Use, Sylke Waibel: diabetes. Evidence-based diagnosis and therapy. 10th edition. Kitteltaschenbuch, Sinsheim 2015, ISBN 978-3-00-050903-2 , p. 160 f.
- ↑ a b c d Red List Online: Information for healthcare professionals NovoNorm ® (repaglinide), accessed on September 22, 2008.
- ↑ a b c Red List Online: Information for professionals Starlix ® (nateglinide), accessed on September 22, 2008.
- ↑ Resolution of the Federal Joint Committee on an amendment to the Drugs Directive (AM-RL): Glinide for the treatment of type 2 diabetes mellitus (PDF; 28 kB) from June 17, 2010.
- ^ Letter from the Federal Ministry of Health to the G-BA (PDF; 179 kB) on August 12, 2010.
- ↑ Glinide can still be used in diabetes therapy ( memento of the original from October 8, 2013 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , Press release diabetes.de March 2011, accessed February 15, 2012.
- ↑ G-BA press release of February 18, 2016, accessed on April 13, 2016 .