Hemetsberger indole synthesis
The Hemetsberger-Indole-Synthesis , or Hemetsberger-Knittel-Indole-Synthesis is a name reaction of the organic chemistry . It describes the thermal decomposition of a 2- azido cinnamic acid ester into an indole -2- carboxylic acid ester .
It was named after Helfried Hemetsberger and Dierk Knittel, who reported on it for the first time in 1969.
Overview reaction
When heated, the 2-azidocinnamate 1 is rearranged, and the 2- indole-2-carboxylic acid ester 2 is obtained as the product .
The radical R is an alkyl radical .
The blue marked bond in the product is the newly formed carbon-nitrogen bond, which leads to ring closure.
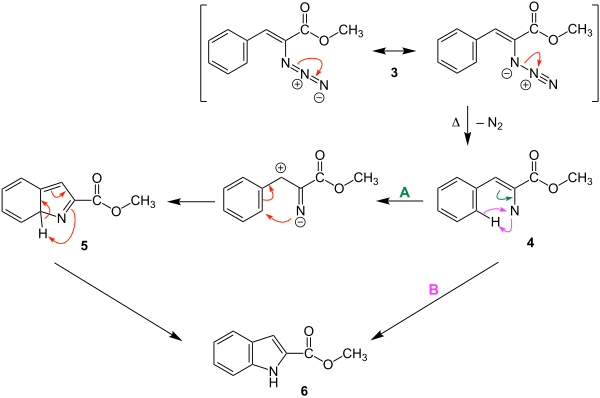
Reaction mechanism
The reaction mechanism presented below is described in the literature and exemplified using methyl 2-azidocinnamate. It has not yet been fully scientifically proven, but it is a plausible process. It is assumed that the synthesis takes place via a nitrene as an intermediate stage.
In the case of the mesomerism-stabilized methyl 2-azidocinnamate 3 , a nitrogen molecule is split off when heated and the nitrene 4 is formed. This can lead to two different electron rearrangements, which are indicated by the colored electron shift arrows.
In path A, the electron rearrangement initially gives molecule 5 . In this there is a [1,5] shift of a proton, the indole-2-carboxylic acid methyl ester 6 is obtained as the product .
In route B, the electron rearrangements in molecule 4 lead directly to ring closure and the methyl indole-2-carboxylate 6 is obtained.
Individual evidence
- ^ A b Zerong Wang: Comprehensive Organic Name Reactions and Reagents , Wiley, 2010, ISBN 978-0-470-63885-9 , pp. 1375-1378, doi: 10.1002 / 9780470638859 .
- ↑ H. Hemetsberger, D. Knittel and H. Weidmann: Synthesis of α-azidocinnamic acid esters In : months booklet for chemistry 100, 1969, p. 1599, doi: 10.1007 / BF00900176 .
- ↑ D. Knittel: Improved synthesis of α-azidocinnamic acid esters and 2H-azirines In: Synthesis 2, 1985, pp. 186-188, doi: 10.1055 / s-1985-31149 .