Hexamethyl tungsten
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Hexamethyl tungsten | |||||||||
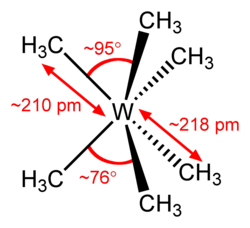
| Molecular formula | W (CH 3 ) 6 | |||||||||
| Brief description |
dark red solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 274.1 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Hexamethyl tungsten is a chemical compound of tungsten from the group of organometallic compounds .
Extraction and presentation
Hexamethyl tungsten can be obtained by reacting tungsten (VI) chloride with trimethyl aluminum .
The preparation by reaction of tungsten (VI) chloride and methyllithium is possible, but unsatisfactory because of the difficult to control and reproducible reaction conditions.
properties
Hexamethyl tungsten is a dark red, very volatile, crystalline solid that begins to slowly decompose from −40 ° C, but can be stored below this temperature for a longer period of time. Its solutions can survive a few days at −25 ° C without noticeable decomposition and can even be used for a short time at room temperature.
Individual evidence
- ↑ a b c d e Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1916.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
