I cell disease
The I-cell disease , also known as mucolipidosis II called, is a very rare autosomal recessive inherited lysosomal storage disease .
| Classification according to ICD-10 | |
|---|---|
| E77.0 | Defects in the post-translational modification of lysosomal enzymes - mucolipidosis II [I-cell disease] |
| ICD-10 online (WHO version 2019) | |
discovery
In 1967, Leroy and DeMars first described the I-cell disease, which is similar to type I mucopolysaccharidosis (Hurler's disease). However, the patients showed no mucopolysaccharide excretion. For this, the two researchers were able to in the fibroblasts of the skin (inclusion Engl. : Inclusin-cells ) detected. The inclusions gave the disease its English name I-Cell Disease .
The disease is extremely rare. The incidence of the two mucolipidoses II and III together is approx. 0.3: 100,000.
root cause
The cause of the disease is a lack of activity of the enzyme N-acetylglucosaminyl-1-phosphotransferase (EC 2.7.8.17), which means that a large number of lysosomal enzymes cannot get into the interior of the lysosomes. The targeting of lysosomal enzymes is disturbed by a defect in phosphotransferase, which normally enables the synthesis of a specific sorting signal. The labeling with mannose-6-phosphate cannot take place and the lysosomal enzymes can no longer be sorted. They reach the extracellular matrix in an uncontrolled manner via the plasma membrane .
Symptoms
The symptoms of the disease are usually already visible at birth, at the latest a few months after birth. The symptoms are similar to those of Hurler's syndrome ( mucopolysaccharidosis I), but are usually observed earlier. In addition, the patients show no mucopolysaccharide secretions. The symptoms show a great variety of variations from family to family and also within a family.
According to Kornfeld and Sly, the following clinical features are possible:
1. Skeletal
kyphoscoliosis , hip dislocation , club feet , joint contractures , vertebral deformities, short stature , dysostosis multiplex
2. Internal organs
Hepatosplenomegaly , cardiomegaly , cardiac vitia
3. Face and head
Coarsened facial features, exophthalmos , hyperplastic gums , scaphocephaly , open mouth, deeply sunken nose
4. Eyes
corneal opacities, swollen eyelids
5. Skin
Thick, rough skin
6. CNS
Severe psychomotor and mental retardation
diagnosis
The diagnosis can be made through a biochemical determination of the activity of the lysosomal enzymes in the serum . The ratio of intra- and extracellular activity of the lysosomal enzymes and the activity of phosphotransferase in cultivated fibroblasts are further possible laboratory parameters.
The inclusions in the fibroblasts are accumulations of mucopolysaccharides , lipids and oligosaccharides .
genetics
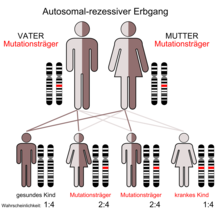
The enzyme N-acetyl-glucosamine-1-phosphotransferase consists of three subunits (alpha, beta and gamma), which are encoded by two different genes . One gene encodes the α and β, the other gene the γ subunit. In the autosomal recessive inherited I-cell disease, the GNPTA gene (GNPTAB) for the α and β subunits on chromosome 12 gene locus q23.3 is mutated. The gene for the γ subunit (GNPTG), on the other hand, is on chromosome 16 gene locus p13.3 and is not affected by the mutation.
therapy
A cure is so far excluded. The treatment is symptomatic.
forecast
The prognosis is extremely poor. Those affected usually do not survive the first decade of life. The cause of death is usually recurrent pneumonia .
Individual evidence
- ↑ JG Leroy, RI De Mars: Mutant enzymatic and cytological phenotypes in cultured human fibroblasts. In: Science . 157/1967, pp. 804-806.
- ^ Society for Mucopolysaccharidoses: Mucolipidosis II / III: Diagnosis ( Memento of December 2, 2011 in the Internet Archive ), accessed on March 26, 2008.
- ↑ A. Hasilik, K. von Figura: Oligosaccharides in lysosomal enzymes. Distribution of high-mannose and complex oligosaccharides in cathepsin D and b-hexosaminidase. In: Eur J Biochem . 121, 1981, pp. 125-129.
- ↑ a b C. Schwab: Transport and modification of lysosomal enzymes in mucolipidosis II / III cells. Dissertation . Philipps University of Marburg, Medicine, 1998.
- ^ A b S. Kornfeld, WS Sly: I-cell disease and pseudo-Hurler polydystrophy: Disorders of lysosomal enzyme phosphorylation and localization. In: The Metabolic and Molecular Bases of Inherited Disease. Vol. II, McGraw-Hill, New York 1995, pp. 2495-2508.
- ^ V. Hieber et al.: Accumulation of [35] -S-mucopolysaccharides in cultured mucolipidosis cells. In: Birth Defects. 6/1975, pp. 307-310.
- ↑ G. Dawson et al .: Glycosphingolipids in cultured human skin fibroblasts. Characterization and metabolism in fibroblasts from patients with inborn errors of glycosphingolipid and mucopolysaccharide metabolism. In: J. Biol. Chem. 247/1972, pp. 5951-5985.
- ↑ GH Thomas et al .: Increased levels of sialic acid associated with sialidase deficiency in I-cell disease fibroblats. In: Biochem. Biophys. Res. Commun. 71/1976, pp. 188-195.
- ↑ S. Tiede et al.: Mucolipidoses. In: Monthly Pediatrics. 154/2006, pp. 955-961.
- ^ S. Tiede et al.: Mucolipidosis II is caused by mutations in GNPTA encoding the alpha / beta GlcNAc-1-phosphotransferase. In: Nat. Med. 11/2005, pp. 1109-1112. PMID 16200072 doi: 10.1038 / nm1305 .
literature
- C. Schwab: Transport and modification of lysosomal enzymes in mucolipidosis II / III cells. Dissertation. Philipps University of Marburg, Medicine, 1998.
- JG Leroy et al: I-cell disease: biochemical studies. In: Pediatr Res . 6/1972, pp. 752-757.
- M. Pavelka, J. Roth: Functional ultrastructure - Atlas of the biology and pathology of tissues. Springer, 2005, ISBN 3-211-83563-6 , pp. 100-101.
- JC Navarro: Pathology of the Nervous System V. Springer, 1991, ISBN 3-540-52873-3 , pp. 145-148.
- S. Tiede: Synthesis of the mannose-6-phosphate recognition marker of lysosomal enzymes: Molecular analysis of the human UDP-N-acetylglucosamine: lysosomal enzyme-N-acetylglucosamine-1-phosphotransferase. Dissertation. University of Hamburg, 2005.