isotherm
Isotherms ( Greek ἴσος ísos 'equal' and θέρμη thérmē 'heat') are lines of the same temperature :
They belong to the isolines and are used in numerous diagrams from all areas of natural and engineering sciences . Alexander von Humboldt coined the idea of isothermal lines in 1817 (initially referred to in French as lignes isothermes or lignes d'égale chaleur ). They are among his suggestions that “have had the most lasting impact on a global scale”.
thermodynamics
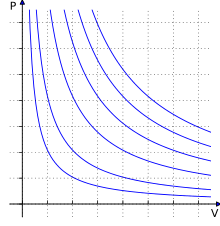
In thermodynamics , isotherms are drawn in, for example, in the pV diagram in order to depict the relationship between pressure p and volume V at constant temperature. If the pressure and volume change and the temperature remains the same, it is an isothermal change of state . In the pV diagram, the work performed by a cycle corresponds to the area that the process curves of the cycle enclose. Two isotherms and two isochors are an example of a cycle .
The isotherm of an ideal gas in the pV diagram can be derived from the thermal equation of state and described by a hyperbola .
Another example of isotherms in thermodynamics are sorption isotherms .
meteorology
In meteorology , isotherms are used to mark areas of the same temperature on meteorological weather maps. The distance between the isotherms gives an indication of the extent of the temperature gradient in an area. This temperature gradient, which can be derived from the arrangement of neighboring isotherms, is an important element in weather forecasting .
Annual isotherms, which are created on the basis of the annual average temperatures, as well as January and July isotherms are common.
Web links
Individual evidence
- ^ Wilhelm Gemoll : Greek-German school and hand dictionary . G. Freytag Verlag / Hölder-Pichler-Tempsky, Munich / Vienna 1965.
- ^ Andreas W. Daum: Alexander von Humboldt . CH Beck, Munich 2019, ISBN 978-3-406-73435-9 , pp. 82 .