Combustion air ratio
A fuel-air mixture, or especially in the case of internal combustion engines , a fuel-air mixture , is characterized by its combustion air ratio λ ( lambda ; also called air ratio or air ratio for short ), a dimensionless characteristic number from the theory of combustion that defines the mass ratio of air to fuel relative to each indicating the stoichiometric ideal ratio for a theoretically complete combustion process . From this key figure, conclusions can be drawn about the combustion process, temperatures , the generation of pollutants and the efficiency .
It is therefore of particular importance in technical areas of application for internal combustion engines and combustion technology , but also in fire theory .
definition
The combustion air ratio sets the actually available air mass in relation to the minimum necessary air mass , which is theoretically required for a stoichiometrically complete combustion:
The limit value 1 is of particular importance for the numerical value:
- If so, the ratio applies as the stoichiometric combustion air ratio with ; this is the case when all fuel molecules can react completely with the oxygen in the air without a lack of oxygen or unburned fuel remaining ( complete combustion ).
- (e.g. 0.9) means "lack of air" (with combustion engines one speaks of a rich or rich mixture)
- (e.g. 1.1) means "excess air" (with combustion engines one speaks of a lean or poor mixture)
Statement: means that 10% more air takes part in the combustion than would be necessary for the stoichiometric reaction. This is also the excess air .
calculation
Approximate calculation using the oxygen content in the exhaust gas:
Approximate calculation using the carbon dioxide content in the exhaust gas:
The maximum concentration is calculated from:
Mass proportions:
Minimum flue gas mass:
Minimum air mass:
Variables:
- Measured content in the exhaust gas
- Gas constant of carbon dioxide =
- Gas constant of nitrogen =
g are the mass proportions of the individual gas in the total mass, the indices denote the gas, RG means proportion of the flue gas (exhaust gas), t means proportion of the dry exhaust gas (before the measurement, the water is very often "filtered" from the exhaust gas, um Avoid falsifications).
- : Minimum air mass required for combustion
Stoichiometric air requirement
The stoichiometric air requirement (also the minimum air requirement ) is a mass ratio of the fuel mass and the associated stoichiometric air mass .
The air requirement can be determined from the mass fractions of a reaction equation, assuming complete combustion of the components.
For common fuels in internal combustion engine construction the following results :
- Petrol ( petrol ): - 14.7 kg of air are required to burn 1 kg of petrol.
- Diesel fuel : - 14.5 kg of air are required to burn 1 kg of diesel fuel.
In naturally aspirated engines, the fresh gas charge at bottom dead center (BDC) always contains a portion of the exhaust gas from the previous work cycle. This residual gas fraction corresponds to the combustion chamber volume at top dead center times the exhaust gas pressure. The gas charge for gasoline engines (air plus exhaust gas) is therefore around 20% higher than with pure air (approx. 1.2 * 14.7 = 17.6 kg of gas per kg of gasoline). Exhaust gas recirculation in gasoline engines also makes its contribution (e.g. 1.4 * 14.7 = 20.6 kg of gas per kg of petrol). Diesel engines are operated with excess air anyway (λ from about 10 at idle to 1.4 (“soot limit”) at full load). Turbo engines can operate the gas exchange without residual gas content (λ = 1.0).
Typical values
Internal combustion engines
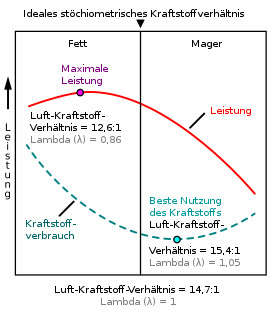
Today's gasoline engines are operated with an air ratio around λ = 1. This enables exhaust gas cleaning with the three-way catalytic converter . A lambda probe in front of the catalytic converter then measures the oxygen content in the exhaust gas and forwards signals to the control unit of the mixture regulator as an element of the engine control unit. The task of the mixture controller is to keep the air ratio close to λ = 1 by varying the injection duration of the individual injection valves . The most efficient operation occurs with a slightly lean mixture of approx. Λ = 1.05. The highest engine output is achieved with a rich mixture of approx. Λ = 0.85. This is also where the highest ignition speed, i.e. reaction speed of the mixture, occurs. Beyond the ignition limits (0.6 <λ <1.6 for gasoline engines), the combustion stops and the engine stops. Diesel engines, on the other hand, work with a lean mixture of λ = 1.3 (at full load, at the soot limit ) and with quality control , i.e. λ is higher at part load and reaches values of up to 6 (when idling, given by the mechanical power loss).
Otto engines are enriched in full load operation. Since the fuel no longer burns completely, the engine and especially the exhaust gas do not get so hot. However, the carbon monoxide and hydrocarbon emissions can then no longer be further oxidized to carbon dioxide and water in the three-way catalytic converter.
Thermal baths and boilers
The measurement of the combustion air ratio of boilers or boilers is part of a flue gas measurement . Fan burners get by at full load with λ = 1.2, atmospheric burners at full load with around λ = 1.4. In part load behavior, the combustion air ratio increases to values of λ = 2 to 4, which leads to an increase in the exhaust gas loss and at the same time to a deterioration in the efficiency.
Gas turbines and jet engines
In gas turbines and jet engines based on them , the combustion takes place inside the combustion chamber at the flame holder close to λ = 1, the subsequent supply of secondary air increases the values to λ = 5 and more. The air ratio is so high because the maximum temperature in the combustion chamber (up to 1600 ° C) and the maximum inlet temperature in the turbine (up to 1400 ° C) must not be exceeded.
See also
literature
- Hans Dieter Baehr: Thermodynamics . Springer-Verlag, Berlin / Heidelberg 1988, ISBN 3-540-18073-7 .
Web links
- RP Energy Lexicon (accessed April 9, 2020)
- Analytical modeling of the operating behavior of a gas engine with the new gas pilot injection method for high power density (accessed on April 9, 2020)
- Developing the efficiency potential of supercharged gasoline engines by means of charge dilution (accessed on April 9, 2020)
- Investigation of potential for improvement with regard to consumption and torque in gasoline engines with the help of 1-dimensional simulation calculation (accessed on April 9, 2020)
- Contribution to the determination of the heat transfers in the combustion chambers of internal combustion engines with homogeneous and partially homogeneous energy conversion (accessed on April 9, 2020)
Individual evidence
- ↑ Klaus Schreiner: Basic knowledge of the internal combustion engine: Questions - calculate - understand - exist . Springer, Wiesbaden 2014, ISBN 9783658061876 , p. 112.