Low-Energy Electron Diffraction
The English term Low-Energy Electron Diffraction ( LEED , dt. "Diffraction of low-energy electrons on surfaces") describes a physical method for examining the arrangement of atoms on surfaces and in thin films. The fundamental effect of the interference of waves is used, which leads to the formation of diffraction patterns which are made visible on an observation screen. The first evidence of the wave nature of the electron radiation succeeded Davisson and Germer in 1927 on a nickel - single crystal at the Bell Laboratories . LEED is used in surface chemistry .
LEED uses electrons with a De Broglie wavelength in the range of atomic distances, around 0.1 nm. The relationship with the energy of the electron beam is
Suitable energies are therefore in the order of 100 eV. At this energy, the penetration depth of the elastically scattered electrons is small, in the range from 0.5 to 1 nm, which makes the method very surface-sensitive. Ultra-high vacuum (UHV) is used to keep the surface atomically clean .
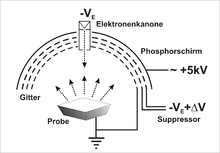
The LEED apparatus
A hot filament, or better a sharp point - cold field emission provides a narrower energy distribution - electrons are emitted and accelerated through an anode towards the sample. The electron beam is focused by an electrostatic lens system. Lateral resolutions are in the μm to mm range. After the electrons have been scattered on the sample , the diffracted electrons pass a grid in front of the fluorescent screen at the (ground) potential of the sample holder, which prevents the diffraction pattern from being distorted, and a retarding grid, on which the inelastically scattered electrons are reflected. The elastically scattered electrons pass and are accelerated onto the screen. The acceleration voltage of several kilovolts ensures a bright picture. Against the penetration of the acceleration voltage, for a better energy resolution, the retarding grid is designed twice.
LEED diffraction pattern
The LEED pattern ideally consists of sharp points that are arranged symmetrically. The electron gun is very often placed in the middle in front of the screen and therefore covers the very bright 0th order reflex when the electrons fall perpendicularly on the sample.
LEED diffraction pattern of silicon carbide at 170 eV
94 eV LEED diffraction pattern of a (100) platinum-rhodium surface covered with CO .
From the position of the reflections only the shape and size of the 2D unit cell result, see Ewald sphere . Since superstructures (by reconstruction or from adsorbates) are imaged in addition to the substrate, different diffraction patterns can be observed depending on the preparation.
The intensities of the reflections vary significantly with the energy of the electrons. To determine the structure, the patterns are recorded with a camera at many energies and the intensity of reflections is determined from the recordings. The structure of the surface can be determined from these intensity curves by comparing the experimental intensity curves with calculated ones. The more reflections that are recorded and the larger the energy range, the more precise the structure determination will be. The calculations are based on a structural model and are complex because multiple scattering must be taken into account. These calculations are called dynamic LEED.
Medium energy electron diffraction
In medium energy electron diffraction (MEED), the multilayer surface growth is observed as a function of time with electron diffraction. If the layers grow monolayer by monolayer on the surface ( Frank van der Merve growth ), then the degree of order of the surface changes periodically. In completely closed layers, the long-range order is greatest, including the intensity of the diffraction reflex. As a result, more or less diffraction reflections are obtained at certain intervals, which indicate the monolayer growth as a function of time.
Web links
- LEEDpat4 (Free LEED simulation software)
Individual evidence
- ^ C. Davisson, LH Germer: Diffraction of Electrons by a Crystal of Nickel . In: Physical Review . tape 30 , no. 6 , 1927, pp. 705-740 , doi : 10.1103 / PhysRev.30.705 .
- ^ MA Van Hove; WH vineyard; CM Chan (1986). Low-Energy Electron Diffraction . Springer-Verlag, Berlin Heidelberg New York. ISBN 3-540-16262-3 . doi: 10.1002 / maco.19870380711 .