Marshalk reaction
The Marschalk reaction is a name reaction in organic chemistry that was discovered in 1936 by Charles Henri Marschalk (1885–1968). It is the reaction of a phenolic anthraquinone to a substituted phenolic anthraquinone.
Overview reaction
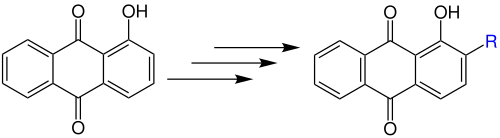
The Marschalk reaction takes place in several reaction steps. A phenolic anthraquinone is substituted by an aldehyde in the ortho position to the phenolic hydroxyl group:
Instead of the hydroxy group , it can also be an anthraquinone with an amino group :
Reaction mechanism
In the proposed reaction mechanism, anthraquinone 1 is initially in redox equilibrium with its anthraquinol 2 . This molecule is now deprotonated by a hydroxide ion. The enolate ion 3 formed now attacks the aldehyde nucleophilically as in an aldol addition . The alcoholate 4 is formed , which is protonated. This creates the aldol 5 . After an aldol condensation, the acidic hydrogen atom is deprotonated by a base. A CC double bond is formed and a hydroxide ion is released as a leaving group . Finally, the intermediate 6 is reoxidized to anthraquinone and the substituted product 7 is formed .
Sample reaction
The starting anthraquinone can optionally be additionally substituted. The Marschalk reaction takes place with various aldehydes. This is the case, for example, with the following reaction with formaldehyde :
modification
The Marschalk reaction can also be carried out twice on one molecule, so that two different side chains are added. For this, however, the side chains must be added in the correct order, since under normal reaction conditions no higher aliphatic or aromatic aldehyde can be introduced as a second vicinal alkyl group. As a result, a selective monoalkylation is also possible with the Marschalk reaction.
The use of different aldehydes was investigated to test whether steric hindrance plays a role in the Marschalk reaction. So differences could be found under the same reaction conditions, but the steric hindrance z. B. be overcome with pivalaldehyde by a higher reaction temperature .
application
The Marschalk reaction can be used to produce derivatives such as 4-demethoxydaunomycinone, which are required for the chemotherapy of cancer.
Individual evidence
- ↑ a b L. A. Mitscher, Tzay-Shiang Wu, Ish Khanna: A useful extension of the Marschalk reaction directed toward synthesis of 11-deoxydoxorubicin antitumor antibiotics. In: Tetrahedron Letters . tape 24 , no. 44 , January 1, 1983, pp. 4809-4812 , doi : 10.1016 / S0040-4039 (00) 94013-9 ( sciencedirect.com [accessed May 22, 2019]).
- ↑ C. Marschalk et al., Bulletin de la Société Chimique de France 3, 1545 (1936)
- ↑ a b c Ellerd, Michael G., Favaloro, Frank G .: Name reactions and reagents in organic synthesis . 2nd ed. Wiley, Hoboken, NJ 2005, ISBN 0-471-22854-0 , pp. 410 .
- ↑ a b Karsten Krohn, Wolfgang Baltus: Synthesis of rac and de-fridamycin E . In: Tetrahedron . tape 44 , no. 1 , January 1, 1988, p. 49-54 , doi : 10.1016 / S0040-4020 (01) 85091-2 ( sciencedirect.com [accessed May 28, 2019]).
- ↑ K. Krohn: Synthesis of anthracyclinones by electrophilic and nucleophilic addition to anthraquinones . In: Tetrahedron . tape 46 , no. 2 , January 1, 1990, p. 219-318 , doi : 10.1016 / S0040-4020 (01) 85414-4 ( sciencedirect.com [accessed May 28, 2019]).
- ↑ AB Argade, AR Mehendale, NR Ayyangar: Marschalk reaction approach for a simple synthesis of (±) 4-demethoxydaunomycinone . In: Tetrahedron Letters . tape 27 , no. 30 , January 1, 1986, pp. 3529-3532 , doi : 10.1016 / S0040-4039 (00) 84841-8 ( sciencedirect.com [accessed May 28, 2019]).