Method of reachable areas
The method of the reachable areas (ger .: Attainable Region Method , AR) is a graphical method of chemical reaction engineering to determine the concentration space that for a given chemical reaction can only be achieved by reacting and mixing. The graphical illustration of the reachable area allows the determination of the optimal reactor network for a given kinetics and a given feed stream .
History of origin
The traditional approach to design and optimization of reactor networks is to test a new reactor configuration against a known one. If a higher yield is achieved, the new reactor configuration and the corresponding process parameters are adopted, otherwise another configuration must be tested. The procedure is trial and error and is time-consuming and costly. Theoretical reactor network optimization often requires a large number of coupled equations. Octave Levenspiel tried to simplify the situation through graphic analysis. Levenspiel's method remained limited in its applicability, as it could only optimize simple reaction problems.
For process optimization, it is paramount to find the most promising solution to a real problem in the shortest possible time. For this purpose, the basic idea of the accessible area was first presented by F. Horn in 1964. He defined the attainable region, i.e. the reachable area, as the amount of all concentrations in the concentration space that can be achieved for a given kinetics and a given inflow by the two operations reacting and mixing.
A graphical method for determining the reachable area was first introduced by Glasser and Hildebrandt in 1987 and gradually expanded. To do this, they assume that the extreme points of the reachable area can always be reached by interconnecting the three reactor types stirred tank reactor (CSTR), flow tube (PFR) and flow tube with side feed (DSR). In recent years, extensions of the method for higher dimensions (Rooney and Glasser, 2000; Abraham and Feinberg, 2004) and for reactive distillation (Agrawal and Mahajani, 2008) have been published.
Determination of the reachable area
One of Feinberg and Hildebrandt's assumptions is that in two-dimensional space the flow pipe (PFR) and the stirred tank reactor (CSTR) are sufficient to form the reachable area. The flow tube and the stirred tank represent the two extreme types of mixing, ideal mixing and no mixing . In the three-dimensional space, the flow pipe with intermediate feed (DSR) has to be added to form the reachable area. This reactor is the intermediate type between the two extremes.
Based on your considerations, Glasser, Feinberg and Hildebrandt formulated the necessary conditions that an area in the concentration area must meet in order to be the accessible area. These conditions are:
- The inflow concentration must be maintained in the area.
- The area is convex because concave areas can be achieved by mixing.
- The reaction vectors on the edge of the area must not point outwards. If the reaction vector points outwards, the reachable area can be enlarged with a connected flow pipe.
- No inverse reaction vector may cut the area from the outside. If this is the case, the reachable area can be enlarged by switching to a stirred tank.
At this point it should be pointed out again that these conditions are only necessary conditions. Even if these conditions are met by an area, it cannot be proven that this is really the largest possible area that can be reached for a given kinetics and a given inflow.
In the following, the two operations reacting and mixing as well as the equations for the three elementary reactions are briefly discussed before the determination of the reachable area is explained using an example.
React
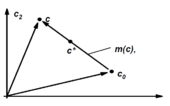
At every point in the concentration space there is a reaction vector r (c) which points in the direction of the reaction at this concentration. If the reaction is described by the differential equation dc = r (c) dλ for a two-dimensional system, the reaction vector can be illustrated as shown in the picture. If one now formulates the differential equation for the two components and forms the ratio of the two, dλ can be canceled out and one obtains the reaction vector. This can be shown at any point on the trajectory .
Mix
When mixing, two concentrations are mixed with one another in a certain ratio α, so that a concentration is created on the straight line between the two concentrations. This allows concave areas of the accessible area to be reached.
Reactor equations
For a stirred tank reactor with ideal mixing, the initial concentration is determined by the algebraic equation as a function of the residence time τ
For an ideal flow pipe, the trajectory of the concentration profile is obtained as a function of the running variable λ by solving the differential equation
The trajectory of the flow pipe with side feed, as an intermediate type of the two previous reactor types, can be determined by solving the differential equation
to be determined.
example
In the following, the determination of the reachable area using the Van de Vusse reaction is shown as an example. The aim of the reactor network should be to maximize the initial concentration of product B.
For the graphical determination of the reachable area, the following assumptions must first be made:
- It is a homogeneous mixture
- There is no change in volume, for example due to mixing
- Isothermal operation is assumed
As the stoichiometry shows, more than two components occur in the Van de Vusse reaction. However, since one is only interested in the course of the trajectories of the educt A and the product B, one can project the actually four-dimensional problem onto a two-dimensional problem.
Construction of the accessible area
In the case of a two-dimensional problem, the first option is to calculate the trajectories for a tubular reactor with increasing reactor length and for a stirred tank reactor with increasing residence time.
If the trajectory of the tubular reactor encloses the trajectory of the stirred tank reactor and the two trajectories do not intersect, the optimal reactor system consists of a single tubular reactor and the area that can be reached can be completed by closing the concave areas with a straight line. These areas can be achieved by back-mixing with the feed stream . Otherwise the trajectory of the stirred tank reactor has to be considered further. This can be extended by a tubular reactor at the points where the reaction vector points outwards (e.g. at the end point of concave areas). Concave areas can be bridged by back-mixing. With this method the complete reachable area can be constructed.
Individual evidence
- ^ O. Levenspiel: Chemical Reaction Engineering , 3rd Ed., John Wiley & Sons, New York (1999)
- ^ F. Horn: Attainable and Non-Attainable Regions in Chemical Reaction Technique in: Proceedings of the third European symposium, Chemical reaction engineering. London, Pergamon, 1964
- ↑ M. Feinberg, D. Hildebrandt: Optimal reactor design from a geometric viewpoint - I. Universal properties of the attainable region , in; Chemical Engineering Science . 1997, 52 (10), 1637-1665.
literature
- Horn (1964): Attainable and non-attainable regions in chemical reaction techniques. Chemical Reaction Engineering, Proceedings of the 3rd European Symposium.
- Glasser, Crowe & Hildebrandt (1987): A geometric approach to steady flow reactors: the attainable region and optimization in concentration space. Industrial & Engineering Chemistry Research.
- Feinberg & Hildebrandt (1997): Optimal reactor design from a geometric viewpoint - I. Universal properties of the attainable region. Chemical Engineering Science.
- Rooney, Hausberger, Biegler & Glasser (2000): Convex attainable region projections for reactor network synthesis. Computers & Chemical Engineering.
- Abraham & Feinberg (2004): Kinetic bounds on attainability in the reactor synthesis problem. Ind. Eng. Chem. Res.
- Agarwal, Thotla & Mahajani (2008): Attainable regions of reactive distillation - Part I. Single reactant non azeotropic systems. Chemical Engineering Science.