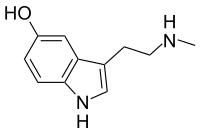
N -methyl serotonin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | N-methylserotonin | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 11 H 14 N 2 O | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| Drug class | ||||||||||
| properties | ||||||||||
| Molar mass | 190.24 g · mol -1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
5-Hydroxy- N -methyltryptamine ( N -methylserotonin for short ), also norbufotenin, is an alkaloid from the tryptamine family. It is a derivative of serotonin . The designation N ω -methylserotonin is also common to distinguish it from tryptamine-derived compounds in which a methyl group is bonded to the nitrogen atom of the indole group .
Occurrence
N -Methylserotonin is found in plants, animals and fungi, including Actaea racemosa (silver grape candle), Zanthoxylum piperitum (Szechuan pepper), Litoria aurea (golden tree frog) and Amanita mushrooms ( amanita mushrooms ).
pharmacology
From a pharmacological point of view, N-methylserotonin binds to several serotonin receptors , including the 5-HT 1A and 5-HT 7 receptors with high affinity ( IC 50 ≤ 2 nM) and selectivity. In addition to serotonergic activity, the compound also acts as a serotonin reuptake inhibitor .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ SL Powell, T. Gödecke, D. Nikolic, SN Chen, S. Ahn, B. Dietz, NR Farnsworth, RB van Breemen, DC Lankin, GF Pauli, JL Bolton: In vitro serotonergic activity of black cohosh and identification of N (omega) -methylserotonin as a potential active constituent. In: Journal of Agricultural and Food Chemistry . Volume 56, Number 24, December 2008, ISSN 1520-5118 , pp. 11718-11726, doi : 10.1021 / jf803298z . PMID 19049296 , PMC 3684073 (free full text).
- ↑ E Yanase, M Ohno, H Harakawa, SI Nakatsuka: Isolation of N , N -dimethyl and N -methylserotonin 5-O-β-glucosides from immature Zanthoxylum piperitum seeds . In: Bioscience, Biotechnology, and Biochemistry . 74, No. 9, 2010, pp. 1951-2. doi : 10.1271 / bbb.100261 . PMID 20834148 .
- ↑ McClean, Stephen; Robinson, Robert C .; Shaw, Chris; Smyth, W. Franklin: Characterization and determination of indole alkaloids in frog-skin secretions by electrospray ionization ion trap mass spectrometry . In: Rapid Communications in Mass Spectrometry . 16, No. 5, 2002, pp. 346-354. doi : 10.1002 / rcm.583 . PMID 11857717 .
- ↑ Tyler, VE, Jr .; Groeger, D .: Amanita alkaloid. II. Amanita citrina and Amanita porphyria . In: Planta Medica . 12, No. 4, 1964, pp. 397-402. doi : 10.1055 / s-0028-1100193 .

