Sodium soap
| Sodium salts of individual fatty acids (examples) |
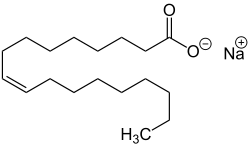
| Sodium oleate, the sodium salt of oleic acid. |
| Sodium palmitate, the sodium salt of palmitic acid. |
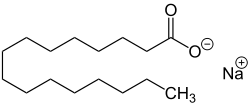
| Sodium stearate , the sodium salt of stearic acid. |
Sodium soap , also out of date soda soap , is a collective term for sodium salts of individual fatty acids or - more often - mixtures of sodium salts of several fatty acids.
Manufacturing
The saponification of natural fats and oils with caustic soda provides mixtures of sodium salts of fatty acids and glycerine . The proportions of the individual fatty acid anions in the mixture of sodium salts depends on the nature and provenance of the triglyceride used as the raw material . A chemically largely uniform sodium soap can be obtained by reacting a pure fatty acid with a stoichiometric amount of sodium hydroxide solution. Examples of such sodium soaps are:
- Sodium oleate, the sodium salt of oleic acid , a white powder, soluble in water and ethanol .
- Sodium palmitate, the sodium salt of palmitic acid (hexadecanoic acid), a white, waxy mass.
- Sodium stearate , the sodium salt of stearic acid (octadecanoic acid), white flake, soluble in water and ethanol.
use
Sodium soaps are used as a component of suppositories , hard soaps ( curd soap ), cosmetics , and as a thickener in mineral oil- based lubricating greases .
More soaps
See also
Individual evidence
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 924.
- ↑ Brockhaus ABC Chemie2 , VEB FA Brockhaus Verlag Leipzig 1965, p 925th
- ↑ a b Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 4: M-Pk. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1985, ISBN 3-440-04514-5 , p. 2745.