Sodium stearate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
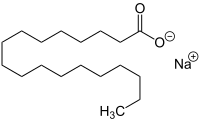
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium stearate | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | C 18 H 35 NaO 2 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 306.46 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.02 g cm −3 |
|||||||||||||||
| Melting point |
205 ° C |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium stearate is the sodium salt of stearic acid and a basic component of many soaps (e.g. curd soap ). Their manufacturing process (reaction of vegetable oils with caustic soda) and, indirectly, the compound itself have been known for a long time. In addition to making cleaning agents, it is also used as a thickener / emulsifier in creams, shampoos and foods.
Extraction and presentation
Sodium stearate can be made for example by saponification of tristearin with sodium hydroxide to sodium stearate, and glycerol .
properties
Sodium stearate is a flammable white solid that is soluble in water.
Individual evidence
- ↑ Entry on SODIUM STEARATE in the CosIng database of the EU Commission, accessed on December 28, 2019.
- ↑ a b c d e Entry on sodium stearate in the GESTIS substance database of the IFA , accessed on February 7, 2019(JavaScript required) .
- ↑ sciencslab.com: Safety data sheet .
- ↑ Chemistrystore: Sodium Stearate safety data sheet ( Memento of March 11, 2006 in the Internet Archive ) (PDF; 126 kB).
- ^ Kremer Pigmente GmbH & Co. KG: Seifen ( Memento from April 26, 2007 in the Internet Archive )
- ↑ University of Applied Sciences Trier: Fettsäuren ( Memento from September 26, 2007 in the Internet Archive ) (PDF; 141 kB).
- ↑ Our products for cosmetics ( Memento from January 6, 2009 in the Internet Archive ).
- ↑ E number directory .
- ↑ seifenparadies.de: What actually is soap? ( Memento from June 26, 2009 in the Internet Archive )
- ↑ University of Basel: Organic Chemistry for Physicians ( Memento of October 3, 2006 in the Internet Archive ) (PDF; 3.5 MB).

