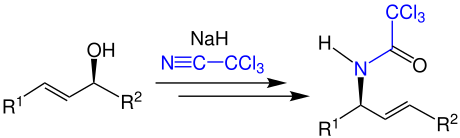Overman rearrangement
The Overman rearrangement is a name reaction in organic chemistry that was named after the American chemist Larry E. Overman (* 1943) after its discoverer . The Overman rearrangement is a variant of the Claisen rearrangement .
Overview reaction
The atom-economical conversion of an allyl alcohol with sodium hydride and trichloroacetonitrile results in an N -allylamide, in the example a trichloroacetic acid amide:
Reaction mechanism
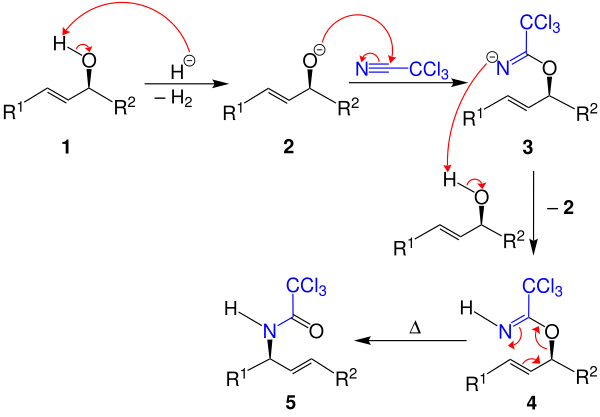
In the first step, the hydroxyl group of the allyl alcohol 1 is deprotonated by a hydride anion generated from the sodium hydride, the alcoholate 2 being formed with the release of hydrogen . The carbon atom of the trichloroacetonitrile is partially positively polarized and is therefore attacked by the formally negatively charged alcoholate 2 . The resulting nitride 3 is protonated by a new allyl alcohol molecule:
This creates the trichloroacetimidate 4 with the formation of a new alcoholate 2 , thus completing the catalytic cycle. This diastereoselective rearrangement is catalyzed in the heat by mercury (II) or palladium (II) salts. The Claisen rearrangement can then take place by heating , the trichloroacetic acid amide 5 being formed. The reaction can also be catalyzed enantioselectively with chiral auxiliaries .
Practical meaning
Allylamine derivatives can be used to make important biological compounds. They can thus serve as base monomers for polyallylamine compounds . These are needed in order to be able to produce the drug sevelamer . Sevelamer is used, among other things, to treat increases in the level of phosphate in the blood . Allylamine is also a building block for the synthesis of a fungicide .
literature
- Jie Jack Li: Name reactions, a collection of detailed reaction mechanism .Vol 1.Springer 2002 . ISBN 3-540-43024-5 .
- T. Nishikawa, M. Asai, N. Ohyabu, M. Isobe: Improved Conditions for Facile Overman Rearrangement , in: J. Org. Chem. 1998 , 63 (1) , 188-192; PMID 11674062 .
- T. Allmendinger, E. Felder, E. Hungerbühler, in: Tetrahedron Lett. 1990 , 31 , 7301-7304.
Individual evidence
- ↑ LE Overman: "Thermal and mercuric ion catalyzed [3,3] -sigmatropic rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions", in: J. Am. Chem. Soc. 1974 , 96 (2) , 597-599; doi : 10.1021 / ja00809a054 .
- ↑ Lane A. Clizbe, LE Overman: Allylically transposed Amines from allylic Alcohols: 3,7-dimethyl-1,6-octadiene-3-amine : In Organic Syntheses . 58, 1978, p. 4, doi : 10.15227 / orgsyn.058.0004 ; Coll. Vol. 6, 1988, p. 507 ( PDF ).
- ↑ LE Overman: "Allylic and propargylic imidic esters in organic synthesis", in: Accounts of Chemical Research 1980 , 13 (7) , 218-224; doi : 10.1021 / ar50151a005 .
- ↑ CE Anderson, LE Overman: "Catalytic Asymmetric Rearrangement of Allylic Trichloroacetimidates. A Practical Method for Preparing Allylic Amines and Congeners of High Enantiomeric Purity", in: J. Am. Chem. Soc. 2003 , 125 (41) , 12412-12413; doi : 10.1021 / ja037086r .
- ↑ Carolyn E. Anderson, Larry E. Overman, and Mary P. Watson: Asymmetric Overman Rearrangement In: Organic Syntheses . 82, 2005, p. 134, doi : 10.15227 / orgsyn.082.0134 ( PDF ).
- ^ LE Overman: "A general method for the synthesis of amines by the rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions", in: J. Am. Chem. Soc. 1976 , 98 (10) , 2901-2910; doi : 10.1021 / ja00426a038 .
- ↑ YK Chen, AE Lurain, PJ Walsh: "A General, Highly Enantioselective Method for the Synthesis of D and L α-amino Acids and allylic Amines", in: J. Am. Chem. Soc. 2002 , 124 (41) , 12225-12231; doi : 10.1021 / ja027271p .