Trichloroacetonitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trichloroacetonitrile | |||||||||||||||
| other names |
Tritox |
|||||||||||||||
| Molecular formula | C 2 Cl 3 N | |||||||||||||||
| Brief description |
colorless to yellow, pungent to caustic smelling liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 144.39 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.44 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
−42 ° C |
|||||||||||||||
| boiling point |
85.7 ° C |
|||||||||||||||
| Vapor pressure |
77 hPa (20 ° C) |
|||||||||||||||
| solubility |
slightly soluble in water |
|||||||||||||||
| Refractive index |
1.441 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Trichloroacetonitrile (Tritox) is a chemical compound from the group of nitriles . As a bifunctional compound, trichloroacetonitrile can undergo reactions both on the trichloromethyl and on the nitrile group. The electron-attracting effect of the trichloromethyl group activates the nitrile group for nucleophilic additions . The high reactivity makes trichloroacetonitrile a versatile reagent , but also causes its sensitivity to hydrolysis .
presentation
The preparation of trichloroacetonitrile by elimination of water from trichloroacetamide was first described in 1873 by L. Bisschopinck from the Katholieke Universiteit Leuven .
Trichloroacetonitrile can be obtained in 54% yield by chlorination of acetonitrile on an activated carbon catalyst impregnated with Zn, Cu and alkaline earth metal halides at 200-400 ° C.
The high temperatures required by this process favor the formation of by-products, such as. B. Carbon tetrachloride . In contrast, the chlorination of acetonitrile saturated with hydrogen chloride leads to pure trichloroacetonitrile in good yields at 50-80 ° C.
Like other halogenated acetonitriles, trichloroacetonitrile is formed from organic substances such as algae, humic acids and proteinaceous material during the disinfecting chlorination of water from natural sources.
properties
Freshly distilled trichloroacetonitrile is a colorless liquid that quickly turns yellow to light brown with a pungent odor and is incompatible with water, acids and bases.
The bond lengths are 146.0 pm (C – C), 116.5 pm (C – N) and 176.3 pm (C – Cl). The bond angle is 110.0 ° (ClCCl).
use
The substitution of all electronegative substituents in the trichloroacetonitrile by nucleophilic attack by alcoholate anions produces orthocarbonic acid esters in high yield . Due to the high reactivity of the chlorine atoms, trichloroacetonitrile - especially in combination with triphenylphosphine - can be used to convert allyl alcohols into the corresponding allyl chlorides.
Acyl chlorides are obtained with carboxylic acids .
Due to the mild reaction conditions, the Cl 3 CCN / PPh 3 system is also suitable for the activation of carboxylic acids and linking with carrier-bound amino compounds to form amides (peptides) in solid-phase syntheses. The corresponding sulfochlorides are formed analogously from sulfonic acids. The activation of diphenylphosphoric acid with Cl 3 CCN / PPh 3 and conversion with alcohols or amines to the corresponding phosphoric acid esters or amides takes place in a similar manner in a gentle and efficient one-pot reaction.
Phenolic hydroxyl groups in nitrogen-containing aromatics can also be converted into the chlorine compounds in this way.
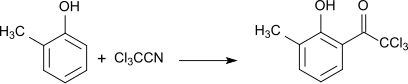
In a Hoesch reaction , substituted phenols react with trichloroacetonitrile to form aromatic hydroxy ketones, e.g. B. from o -cresol the o -state trichloroacyl derivative in 70% yield.
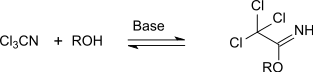
The electron-attracting effect of the trichloromethyl group activates the nitrile group of the trichloroacetonitrile against attack by nucleophilic O- , N- and S -compounds. Thus, under base catalysis with alcohols in direct and reversible addition, O -alkyl trichloroacetimidates are formed, which can be isolated as stable adducts that are not very sensitive to hydrolysis.
With primary and secondary amines, N- substituted trichloroacetamidines are formed in smooth reaction with good yields , which can be purified by vacuum distillation and are obtained as colorless, foul-smelling liquids. The reaction with ammonia and then with anhydrous hydrogen chloride gives the solid trichloroacetamidine hydrochloride, which is the starting compound for the fungicide etridiazole .
The Overman rearrangement is the [3,3] -sigmatropic and diastereoselective rearrangement of a trichloroacetimidate formed from an allyl alcohol and trichloroacetonitrile under base catalysis into an allylamine. Benzyl trichloroacetimidate can be obtained in a simple manner from benzyl alcohol and trichloroacetonitrile. Benzyl trichloroacetimidate is suitable as a benzylation reagent for sensitive alcohols under mild conditions and preservation of chirality .
O -Glycosyl-trichloroacetimidate to activate carbohydrates
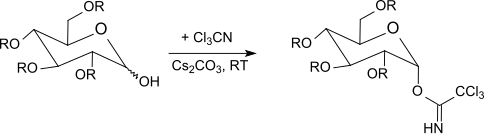
RR Schmidt and co-workers described the selective anomeric activation of O -protected hexopyranoses ( glucose , galactose , mannose , glucosamine , galactosamine ) and of hexofuranoses and pentopyranoses with trichloroacetonitrile in the presence of a base, as well as glycosylations under acid catalysis.
β-Trichloroacetimidates are formed selectively with potassium carbonate as the base under kinetic control, while only α-trichloroacetimidates (thermodynamically controlled) are obtained with sodium hydride , cesium carbonate or potassium hydroxide and in the presence of phase transfer catalysts.
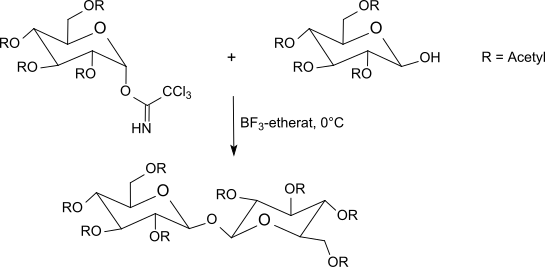
The reaction of trichloroacetimidates with O -protected sugars, which is usually carried out at −40 ° C to room temperature with boron trifluoride etherate in methylene chloride , usually gives better results than the Koenigs-Knorr method using silver salts or the Helferich method with problematic mercury salts . The inversion at the anomeric center leads to β- O- glycosides when using α-trichloroacetimidates . The trichloroacetimidate method often produces sterically uniform glycosides in very good yields under mild reaction conditions.
Even without additional acid catalysis, thioacetic acid reacts with acetyl-protected α-galactosyl trichloroacetimidate to form the thioglycoside, from which 1-thio-β- D- galactose, which is useful for separating racemates from amino acids, is easily accessible after splitting off the protective groups .
In the first half of the 20th century trichloroacetonitrile was an important fumigant , but today it has become obsolete for this application .
Individual evidence
- ↑ a b Frank Bernsdorff: Investigations on the abiotic formation of acetonitrile, haloacetonitriles and trichloronitromethane . GRIN, 2007, p. 5 ( limited preview in Google Book search).
- ↑ a b Data sheet Trichloroacetonitrile, 98% from Sigma-Aldrich , accessed on October 22, 2013 ( PDF ).
- ↑ a b c d e f g h Entry on trichloroacetonitrile in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Entry on trichloroacetonitrile in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ L. Bisschopinck, About the chlorinated acetonitriles , Ber. German chem. Ges. , 6 , (1), 731-734 (1873), doi : 10.1002 / cber.187300601227 .
- ↑ Patent application US2375545 : Process for the preparation of trichloroacetonitrile. Registered October 7, 1943 , published May 8, 1945 , Applicant: Imperial Chemical Industries, Inventor: RT Foster.
- ↑ Patent application US2745868 : Process for the production of trichloroacetonitrile. Registered on February 8, 1954 , published on May 15, 1956 , applicant: Deutsche Gold- und Silber-Scheideanstalt , formerly Roessler, inventor: G. Käbisch.
- ↑ Guidelines for Drinking Water Quality , 3rd edition, Vol. 1, Recommendations, World Health Organization, Geneva, 2004, ISBN 9-2415-4638-7 , PDF .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Structure of Free Molecules in the Gas Phase, pp. 9-46.
- ↑ ED Matveeva et al., Regioselective and stereoselective substitution of hydroxyl group for halogen in allyl alcohols , Zh. Org. Khim., 31 , (8), 1121-1125 (1995).
- ↑ DO Jang et al., A mild and efficient procedure for the preparation of acid chlorides from carboxylic acids , Tetrahedron Lett., 40 , (29), 5323-5326 (1999).
- ↑ J. Vago, J. Greiner, A useful acylation method using trichloroacetonitrile and triphenylphosphine for solid phase organic synthesis , Tetrahedron Lett., 43 , (34), 6039-6041 (2002).
- ↑ O. Chantarasriwong et al., A practical and efficient method for the preparation of sulfonamides utilizing Cl 3 CCN / PPh 3 , Tetrahedron Lett., 47 , (42), 7489-7492 (2006).
- ↑ A. Kasemsuknimit et al., Efficient amidation and esterification of phosphoric acid using Cl 3 CCN / Ph 3 P , Bull. Korean Chem. Soc., 32 , (9), 3486-3488 (2011).
- ↑ W. Kijrungphaiboon et al., Cl 3 CCN / PPh 3 and CBr 4 / PPh 3 : two efficient reagent systems for the preparation of N-heteroaromatic halides , Tetrahedron Lett., 53 , 674-677 (2006).
- ^ R. Martin: Aromatic Hydroxyketones: Preparation and Physical Properties. Vol. 1 Hydroxybenzophenones . 3. Edition. Springer, 2011, ISBN 978-1-4020-9787-4 , doi : 10.1007 / 978-1-4020-9787-4 .
- ↑ JU Nef, Ann. Chem., 287 , 274 (1895).
- ↑ JC Grivas, A. Taurins: Reaction of trichloroacetonitrile with primary and secondary amines . In: Canadian Journal of Chemistry . 36 (5), 1958, pp. 771-774, doi : 10.1139 / v58-113 .
- ^ FC Schaefer, GA Peters, Base-Catalyzed Reaction of Nitriles with Alcohols. A Convenient Route to Imidates and Amidine Salts , J. Org. Chem., 26 , (2), 412-418, (1961), doi : 10.1021 / jo01061a034 .
- ↑ EP Eckenberg et al., A useful application of benzyl trichloroacetimidate for the benzylation of alcohols , Tetrahedron, 49 , 1619-1624 (1993).
- ^ RR Schmidt, J. Michel, Simple Synthesis of α- and β- O -Glycosylimidates. Production of glycosides and disaccharides , Angew. Chem., 92 , 763-764 (1980).
- ^ RR Schmidt, New methods for glycoside and oligosaccharide synthesis - are there alternatives to the Koenigs-Knorr method? , Angew. Chem. 98 : 213-236 (1986).
- ↑ RR Schmidt, W. Kinzy, anomeric oxygen-activation for glycoside synthesis - the trichloroacetimidate method , Adv Carbohydr.. Chem. Biochem., 50 , 21-123 (1994).
- ^ RR Schmidt, K.-H. Jung, O ligosaccharide synthesis with trichloroacetimidates , In: Preparative Carbohydrate Chemistry, S. Hanessian, Ed., Marcel Dekker, New York, 283-312 (1997), ISBN 0-8247-9802-3 .
- ^ RR Schmidt, J. Michel, Liebigs Ann. Chem. , 1343-1357 (1984).
- ↑ FJ Urban et al., Tetrahedron Lett., 31 , 4421-4424 (1990)
- ↑ VJ Patil, Tetrahedron Lett., 37 , 1481-1484 (1996).
- ↑ A. Jegorov et al., 1-Thio-β-D-galactose as a chiral derivatization agent for the resolution of D , L -aminoacid enantiomers , J. Chromatogr. A, 673 (2): 286-290 (1994).
- ^ NM Sax, RJ Lewis, Hawley's Condensed Chemical Dictionary , 11th ed., Van Nostrand Reinhold, New York, pp. 261, 1175 (1987).