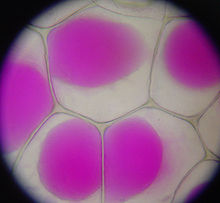
Plasmolysis

In biology, plasmolysis is the shrinkage of the protoplast of a plant cell , with the plasma membrane becoming detached from the cell wall . To do this, the cell must be exposed to a plasmolytic. This is a hypertonic solution; H. a solution that contains plenty of salts or sugar components and thus has more dissolved particles than the cell sap of the vacuole . In this case, water flows osmotically from the vacuole through the membranes ( tonoplast , plasmalemma ) into the surrounding, more concentrated medium, so that the cell sap space becomes smaller and separates the plasma tube adhering to the vacuole together with the plasmalemma from the cell wall. If the plasma does not adhere to the wall, the detachment is rounded (convex plasmolysis); if the wall adheres strongly, bizarre shapes form, in which the plasma is drawn out into thin threads (Hecht's threads) (concave plasmolysis). This process is reversible, i.e. reversible via deplasmolysis (provided that the cell was not damaged by excessive plasmolysis).
The state in which the protoplast releases straight from the cell wall - so the cell no longer turgid , but not yet plasmolyzed right - is called Grenzplasmolyse . This is the case in an isotonic medium.
Cause - osmosis as the mainspring of plasmolysis
If there is another highly concentrated salt solution or another highly concentrated substance outside of cells or tissues, the protoplast will detach itself from the cell wall after a short time. The vacuole becomes smaller until, in extreme cases, the protoplast is completely detached from the cell wall and finally appears as a spherical structure.
root cause
The concentration of dissolved substances in the water is higher (hypertonic) compared to the interior of the cell. Such imbalances automatically cancel each other out in nature (if possible). So water diffuses out of the cell plasma and the cell sap vacuole. The mainspring of this concentration balance that takes place here is osmosis.
The compensation now comes about primarily through the diffusion of water through the cell membrane . All of this is only possible because biomembranes are primarily permeable to water, but not to dissolved substances. The membrane is called semipermeable (semi-permeable) or selectively permeable (only permeable to certain substances). The diffusion of water through a selectively permeable membrane is also called osmosis.
The hypotonic (i.e. containing less dissolved particles) solution in the cell loses water, as the water particles diffuse outwards into the hypertonic (more highly concentrated) solution. As the dissolved water particles decrease, the vacuole and cytoplasm inside the cell shrink.
From this it follows: The addition of a hypertonic solution to the cell (during plasmolysis) actually increases the salt concentration inside the cell, which thus assimilates the concentration of the external medium (and this is additionally diluted by the water flowing out of the cell) is reversible; If the cell is placed in distilled water, this diffuses back into the cell and there dilutes the solution: deplasmolysis.
If both solutions, the external medium and the inside of the cell, have the same concentration of a dissolved substance, then it is an isotonic solution. However, osmosis also takes place with these, since osmosis is a permanent process. In contrast to hypertonic and hypotonic solutions, however, the water released by the cell is equal to the water uptake. So it is a constant flow equilibrium (also called dynamic equilibrium) between the isotonic solutions of the cell and the external medium.
Physical approach
At the particle level, the process could be described as follows: The salt ions (e.g. Na + and Cl - , i.e. sodium and chloride ions) form a hydration shell around them. This means that the polar water molecules arrange themselves accordingly around the ions and are thus immobilized. This means that the probability that a water molecule from the salty (hypertonic) solution diffuses through the membrane is lower than the other way around. In the less concentrated solution (in plasmolysis: in the vacuole) the water molecules are therefore more mobile. Therefore they diffuse more easily through the membrane to the other side out of the vacuole.
Web links
- Plasmolysis: animation and explanation
- Video: plasmolysis and cytorrhysis . Institute for Scientific Film (IWF) 1974, made available by the Technical Information Library (TIB), doi : 10.3203 / IWF / C-1144 .