Post-translational protein transport
In biology, post-translational protein transport describes a mechanism that transports proteins through a membrane after their synthesis (see translation ). Various forms of post-translational protein transport can be found in pro- and eukaryotic cells. If the (partial) transport of proteins takes place during translation, one speaks of cotranslational transport .
properties
Post-translational protein transport takes place primarily in organelles such as mitochondria , plastids (and also their thylakoids ) and peroxisomes as well as in the cell nucleus . Post-translational protein transport is also found on the cytoplasmic membrane of bacteria.
Another form of post-translational transport is found at the endoplasmic reticulum (ER), where proteins are transported from the cytosolic side through the ER membrane into the lumen of the ER.
During this process, the proteins are completely synthesized in the cytoplasm and only then transported through the ER membrane. The majority of the knowledge available today about this form of ER transport comes from studies carried out on the yeast Saccharomyces cerevisiae . The signal sequence of a protein determines whether a secretory protein is co- or post-translationally transported into the ER. In the case of more hydrophilic signal sequences, the affinity of the signal recognition particle (SRP) to the signal sequence seems to be less pronounced, which means that there is no firm binding and thus no translation pause that would initiate cotranslational transport. The protein is completely synthesized in the cytoplasm and detached from the ribosome .
In order to keep the transport substrate in a translocation-competent state, cytosolic chaperones of the Hsp70 family bind to the protein. Little is known about the subsequent process of targeting the protein to the ER membrane. The bound cytosolic factors do not seem to play an essential role for the actual transport through the ER membrane, since transport proteins denatured by urea (and thus freed from HSP70) are transported post-translationally in vitro just as efficiently as the native proteins.
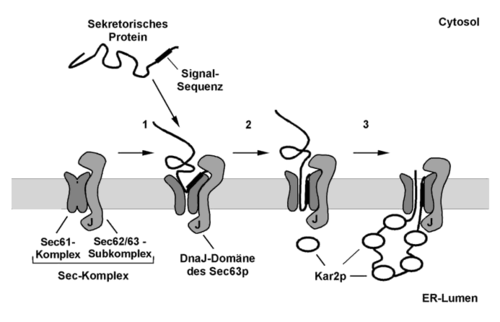
Reconstitution experiments with purified components from yeast microsomes showed that, in addition to the luminal chaperone Kar2p and ATP, a special membrane complex is required to transport prepro-a-factor in vitro post-translationally. This complex is the hetero-heptameric Sec complex , which is composed of the trimeric Sec61 complex and the tetrameric Sec62 / Sec63 complex. Similar to the ribosome with the Sec61 complex, the Sec complex forms ring-shaped structures in the membrane.
The actual post-translational transport of the proteins through the ER membrane takes place in two steps. First, the protein to be transported binds to the Sec complex independently of Kar2p and ATP, whereby the cytosolic components of the Sec62 / Sec63 subcomplex probably form a kind of signal sequence antenna in the membrane. Cross-linking studies have shown that the signal sequence of the protein is recognized and bound by the large subunit of the Sec61 complex during this phase. In the second step of the transport process, the bound polypeptide chain is moved through the channel formed by the Sec61 complex as in cotranslational transport, whereby Kar2p and ATP are also required for efficient transport. Kar2p is an Hsp70 homologous protein that is required for post-translational transport both in vivo and in vitro. The ATP-bound form of Kar2p binds to the DnaJ homologous domain of the Sec63p protein from the Sec62 / 63 subcomplex located in the lumen of the ER and is transferred to the polypeptide chain of the protein to be translocated under ATP hydrolysis with low sequence specificity.
For the model protein prepro-a-factor it could be shown in vitro that the transport through the ER membrane takes place according to the principle of a molecular ratchet . The transport substrate can diffuse freely in both directions as a result of the Brownian molecular movement in the translocon channel. Only when the luminal Kar2p attaches to the polypeptide chain does a directed transport process arise, since areas of the polypeptide chain located in the lumen can no longer slide back into the channel due to the bound Kar2p. Through the successive binding of further Kar2p proteins to the polypeptide chain, the protein is transported into the lumen of the ER.
Model of the post-translational protein transport through the ER membrane of the yeast Saccharomyces cerevisiae .
- The protein to be transported is completely synthesized in the cytoplasm and kept in a translocation-competent state by cytosolic chaperones. The signal sequence of the protein is recognized and bound by the Sec complex in a step that is independent of ATP and Kar2p.
- The N-terminus of the polypeptide chain probably inserts in the form of a hairpin into the translocation channel formed by the Sec61 subcomplex.
- The actual translocation of the polypeptide chain through the ER membrane takes place in an ATP and Kar2p-dependent process. The luminal Kar2p is activated by the DnaJ domain of Sec63p to bind peptides. As soon as the polypeptide chain to be transported emerges on the luminal side of the ER membrane, Kar2p is transferred to these areas with ATP cleavage, which prevents the chain from sliding back (molecular ratchet). Through the successive binding of further Kar2p proteins, the undirected Brownian molecular movement of the polypeptide chain in the translocon is converted into a directed transport process.
Bacterial protein secretion must in Gram-negative bacteria overcome even the outer membrane of the bacteria. To this end, the organisms have developed at least five different systems (type I to type V).
literature
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer : Biochemistry. 6 edition, Spektrum Akademischer Verlag, Heidelberg 2007. ISBN 978-3-8274-1800-5 .
- Donald Voet, Judith G. Voet: Biochemistry. 3rd edition, John Wiley & Sons, New York 2004. ISBN 0-471-19350-X .
- Bruce Alberts , Alexander Johnson, Peter Walter, Julian Lewis, Martin Raff, Keith Roberts: Molecular Biology of the Cell , 5th Edition, Taylor & Francis 2007, ISBN 978-0815341062 .