Pschorr cyclization
The Pschorr cyclization is a name reaction of organic chemistry , which was named after its discoverer, the German chemist Robert Pschorr (1868-1930). It describes the intramolecular substitution of aromatic compounds via aryldiazonium salts as an intermediate stage and is catalyzed by copper . This reaction is a variant of the Gomberg-Bachmann reaction .
Overview reaction
The Pschorr cyclization was originally developed for the production of phenanthrene and phenanthrene derivatives . The following reaction scheme shows an example of the synthesis of phenanthrene by means of the Pschorr cyclization:
Reaction mechanism
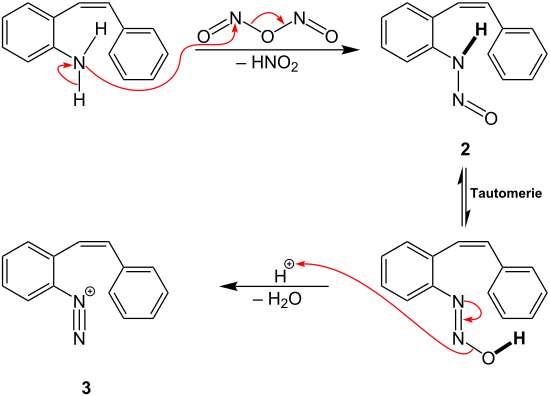
In the course of the Pschorr cyclization, the starting compound is initially diazotized , so that an aryldiazonium salt is obtained as an intermediate. For this purpose, sodium nitrite is added to hydrochloric acid in advance of the reaction in order to obtain nitrous acid . The nitrous acid is then protonated and reacted with a further equivalent of nitrous acid. This results in intermediate 1 , which is later used to diazotize the aromatic amine :
A possible reaction mechanism for the Pschorr cyclization is now to be described in accordance with the overview reaction :
Intermediate 1 is used to substitute a hydrogen atom from the amino group of the starting compound. A nitroso group is introduced as a new substituent . In addition, nitrous acid is split off and intermediate stage 2 is produced. This then reacts via tautomerism and elimination of water to form the aryldiazonium cation 3 .
Through the use of the copper catalyst will now nitrogen from aryl diazonium cation 3 cleaved, so that a aryl - radical forms that has a ring closure to give the intermediate 4 reacts. Finally, using the copper catalyst again, the rearomatization takes place and phenanthrene is obtained.
Atomic economy
The Pschorr cyclization has a relatively good atom economy , since essentially only nitrogen occurs as waste material. Two equivalents of nitrous acid are used for diazotization, one equivalent being formed back in the course of the reaction. The use of copper is catalytic and therefore does not have a negative effect on the atomic efficiency of the reaction. When considering economic aspects, however, it should also be noted that the Pschorr cyclization often only gives low yields .
See also
Individual evidence
- ↑ Robert Pschorr: New synthesis of phenanthrene and its derivatives . In: Reports of the German Chemical Society . tape 29 , no. 1 , 1896, p. 496-501 , doi : 10.1002 / cber.18960290198 .
- ↑ a b Ivica Cepanec: Synthesis of biaryls . 1st ed.Elsevier, Amsterdam 2004, ISBN 978-0-08-044412-3 , pp. 25-27 .
- ↑ a b Jie Jack Li: Name reactions: A collection of detailed mechanisms and synthetic applications . 5th ed.Springer, Cham 2014, ISBN 978-3-319-03979-4 , pp. 499-500 , doi : 10.1007 / 978-3-319-03979-4 .