Gomberg-Bachmann reaction
The Gomberg-Bachmann reaction is a name reaction in organic chemistry , which was named after the Ukrainian-US chemist Moses Gomberg (1866-1947) and the US chemist Werner Emmanuel Bachmann (1901-1951) . The reaction was developed in 1924 for the synthesis of symmetrical and asymmetrical diaryls ( biphenyls ) from aryl diazonium salts .
Overview reaction
In the Gomberg-Bachmann reaction, aryldiazonium salts react in an aqueous, alkaline solution with aromatics (e.g. benzene) to form diaryl compounds:
The intramolecular variant of the Gomberg-Bachmann reaction is known as the Pschorr cyclization .
mechanism
The mechanism for the synthesis of biphenyl will be explained using the example of benzene diazonium chloride and benzene . If diazonium salt 1 is treated with sodium hydroxide solution, benzene diazo hydroxide 2 is formed in an equilibrium reaction , which reacts with another diazonium salt to form benzene diazo anhydride 3 with elimination of protons . Rüchardt provided the proof for the existence of 3 through cross-breeding experiments . This anhydride splits off nitrogen and reacts to form a benzene diazo anhydride radical 4 and a phenyl radical 5 . The reactive phenyl radical attacks the benzene with the formation of a mesomeric stabilized radical (phenylcyclohexadienyl radical) ( 6 ). This reaction step is a radical substitution . The radical 6 then reacts with 4 , splitting off 2 to form biphenyl ( 7 ).
Due to side reactions, the Gomberg-Bachmann reaction often has a yield of less than 40%. By phase transfer catalysts , the yield can be significantly increased.
selectivity
According to the general rule that the selectivity decreases with increasing reactivity, the highly reactive aryl radicals are not very selective. That is why benzene is usually used as an aromatic.
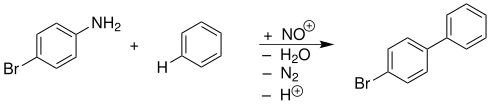
example
4-bromoaniline and benzene react by diazotization in an alkaline solution to form 4-bromobiphenyl:
See also
Individual evidence
- ^ M. Gomberg , WE Bachmann : The Synthesis of Biaryl Compounds by means of the Diazo Reaction . In: J. Am. Chem. Soc. tape 46 , 1924, pp. 2339-2343 , doi : 10.1021 / ja01675a026 .
- ↑ T. Laue, A. Plagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 3-8351-0091-2 , p. 158-160 .
- ↑ DeLos F. DeTar: The Pschorr Synthesis and Related diazonium Ring Closure Reactions . In: Organic Reactions . tape 9 , 1957, pp. 409-462 , doi : 10.1002 / 0471264180.or009.07 .
- ↑ T. Laue, A. Plagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 3-8351-0091-2 , p. 158-160 .
- ^ Z. Wang: Comprehensive Organic Name Reactions and Reagents . Vol. 1. Wiley, 2009, ISBN 978-0-471-70450-8 , pp. 1248-1251 .
- ↑ Christoph Rüchardt, Ekkehard Merz: The mechanism of the Bachmann-Gomberg reaction . In: Tetrahedron Letters . tape 5 , no. 36 , 1964, pp. 2431-2436 , doi : 10.1016 / S0040-4039 (00) 70404-7 .
- ↑ Jan Bülle, Aloys Hüttermann: The basic knowledge of organic chemistry . Wiley-VCH, 2008, ISBN 978-3-527-30847-7 .
- ^ M. Gomberg , WE Bachmann : The Synthesis of Biaryl Compounds by means of the Diazo Reaction . In: J. Am. Chem. Soc. tape 42 , 1924, pp. 2339-2343 , doi : 10.1021 / ja01675a026 .
- ↑ MB Smith, J. March: March's Advanced Organic Chemistry . Wiley, 2001, ISBN 0-471-58589-0 .
- ^ R. Bolton, G. Williams: Homolytic arylation of aromatic and polyfluoroaromatic compounds . In: Chem. Soc. Rev. Band 15 , 1986, pp. 261-289 , doi : 10.1039 / CS9861500261 (review).
- ↑ James R. Beadle, Stephen H. Korzeniowski, David E. Rosenberg, Blanche J. Garcia-Slanga, George W. Gokel: Phase-transfer-catalyzed Gomberg-Bachmann synthesis of unsymmetrical biarenes: a survey of catalysts and substrates . In: The Journal of Organic Chemistry . tape 49 , no. 9 , 1984, pp. 1594-1603 , doi : 10.1021 / jo00183a021 .
- ↑ S. Hauptmann: Reaction and Mechanism in Organic Chemistry . Teubner Verlag, 1991, ISBN 978-3-519-03515-2 .
- ↑ M. Gomberg , WE Bachmann : p-Bromobiphenyl In: Organic Syntheses . 8, 1928, p. 42, doi : 10.15227 / orgsyn.008.0042 ; Coll. Vol. 1, 1941, p. 113 ( PDF ).