Biaryls
| Biaryls |
|
Biphenyl (not: diphenyl) |
| 2-phenylphenol |
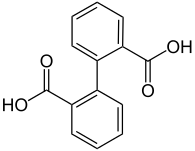
| Diphenic acid |
| Biphenyl-4,4'-dicarboxylic acid |
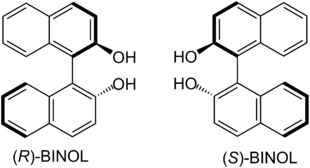
| BINAP enantiomers have axial chirality. |
| BINOL enantiomers have axial chirality. |
As biaryls (not: diaryls) refers to compounds in which two aryl groups are linked together via a single bond. The simplest biaryl is biphenyl .
synthesis
The synthesis can be done by different methods:
- Ullmann reaction based on aryl halides and copper powder. Mainly symmetrical biaryls are produced in this way.
- Cross couplings ( Hiyama coupling , Kumada coupling , Negishi coupling , Stille coupling , Suzuki coupling ) allow the targeted synthesis of unsymmetrical biaryls.
use
The production of biaryls by means of cross-coupling is an important tool in preparative organic chemistry and is of particular importance in the synthesis of active ingredient libraries in order to investigate structure-activity relationships (SAR). Pure enantiomers of BINOL and BINAP - as well as corresponding derivatives - have gained importance as enantioselective catalysts in the targeted production of enantiomerically pure natural products and medicinal products . BINOL is used, for example, as a ligand of a lanthanide in the Shibasaki aldol reaction in order to add unmodified aldehydes enantioselectively to ketones . Heterobimetal catalysts derived from BINOL are also used in the enantioselective addition of phosphorus nucleophiles to imines .
Individual evidence
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 1: A-Cl. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1979, ISBN 3-440-04511-0 , p. 426.
- ^ Russell E. Malz: Catalysis of organic reactions. Marcel Dekker, New York 1996, ISBN 0-8247-9807-4 , p. 147 ( limited preview in Google book search).
- ↑ J. Bülle, A. Hittermann: The basic knowledge of organic chemistry . Thieme, Stuttgart 2000, p. 308 ( limited preview in the Google book search).
- ^ H. Gröger , Y. Saida, H. Sasai, K. Yamaguchi, J. Martens , M. Shibasaki : A New and Highly Efficient Asymmetric Route to Cyclic alpha-Amino Phosphonates: The first Catalytic Enantioselective Hydrophosphonylation of Cyclic Imines Catalyzed by Chiral Heterobimetallic Lanthanoid Complexes. In: J. Am. Chem. Soc. 120, 1998, pp. 3089-3103, doi : 10.1021 / ja973872i .
- ^ I. Schlemminger, Y. Saida, H. Gröger, W. Maison, N. Durot, H. Sasai, M. Shibasaki, J. Martens: Concept of Rigidity: How to Make Enantioselective Hydrophosphonylation of Cyclic Imines Catalyzed by Chiral Heterobimetallic Lanthanoid Complexes almost perfect. In: J. Org. Chem. 65, 2000, pp. 4818-4825, doi : 10.1021 / jo991882r .