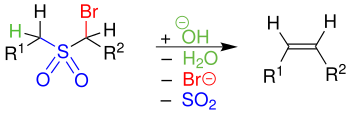Ramberg-Bäcklund reaction
The Ramberg-Bäcklund reaction is a name reaction in organic chemistry that was named after its Swedish discoverers Ludwig Ramberg (1874–1940) and Birger Bäcklund . The reaction is a synthesis for alkenes .
Overview reaction
During the reaction, an α-halogenated sulfone is converted into an alkene by reaction with a strong base .
mechanism
The α-halogenated sulfones (here with the halogen bromine ) are treated with bases. The hydroxide ion is used as the base , which attacks the proton and thus creates a carbanion . The carbanion splits off the halide through a nucleophilic substitution . An episulfone intermediate is formed. After sulfur dioxide was split off by a so-called cheletropic reaction, an olefin or alkene was formed. The more unstable ( Z ) -alkene is predominantly formed .
The α-halogenated sulfones can be obtained from thioethers by reaction with a halogenating agent such as N -chlorosuccinimide and subsequent oxidation on the sulfur. In addition to the aqueous solutions of alkali hydroxides , alcoholates and potassium tert-butoxide in an ether are also used as bases .
use
The Ramberg-Bäcklund reaction is z. B. used for the synthesis of strained unsaturated rings.
Individual evidence
- ↑ T. Laue, A. Plagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 3-8351-0091-2 , p. 279-281 .
- ↑ L. Ramberg, B. Bäcklund, Ark. Chim., Mineral Geol. , 1940 , 27 Vol 13A, 1-50.
- ↑ T. Laue, A. Plagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 3-8351-0091-2 , p. 279-281 .