Rothemund reaction
The Rothemund reaction (also called Rothemund synthesis ) is a name reaction in organic chemistry that was first described in 1936 by Paul Rothemund. With the help of this synthesis meso- substituted porphyrins can be produced.
Overview reaction
In the Rothemund reaction, four equivalents of pyrrole 1 react with four equivalents of an aldehyde 2 with the help of an acid ( hydrochloric acid was used by Rothemund) as a catalyst and subsequent oxidation to form the meso- substituted one in the α, β, γ and δ positions Porphyrin derivative 3 .
General
The Rothemund reaction paved the way for the synthesis of many different porphyrins. Rothemund's team had already obtained positive results in the synthesis of porphyrins with 25 different aliphatic , aromatic and heterocyclic aldehydes . Pyrrole is required for the reaction , as well as hydrochloric acid as a catalyst . As solvents was methanol used. The reaction takes place over a period of 24 hours at 140-150 ° C. The synthesis was modified again by Alan D. Adler's team in 1964. In 1987 the procedure was improved once more by Jonathan S. Lindsey and others.
Reaction mechanism
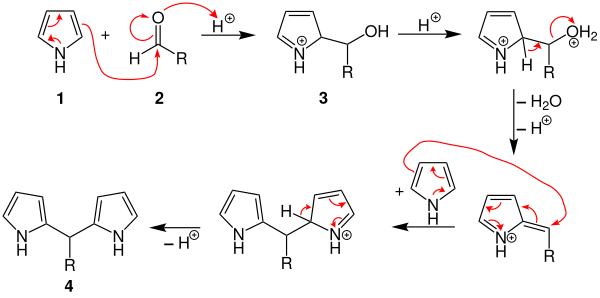
In the first step, the aldehyde 1 is protonated in an acidic environment and attacked by the pyrrole 2 in a nucleophilic manner . The alcohol 3 formed is protonated and water is split off. The next step is another nucleophilic attack by a pyrrole molecule and the intermediate dipyrromethane 4 is formed .
What happens after the formation of dipyrromethane is controversial. Two options are shown below. The dipyrromethane reacts further to give the tetrapyrromethane derivative 5 according to the reaction mechanism described above . Then there is an intramolecular nucleophilic attack and thus cyclization . This creates the porphyrinogen derivative 6 .
Another possibility would be that two equivalents of dipyrromethane react further together with two equivalents of the aldehyde used to form the porphyrinogen derivative 6 .
By oxidation of Porphyrinogenderivat 6 formed as the product porphyrin derivative 7 .
application
The low yield of less than 5% in the production of porphyrins using this method is the reason why this process, as described by Rothemund, is not suitable for industrial use. Adler's team was able to obtain a yield of around 20% with slight modifications. With further modifications they even managed to get 35–40% yield, but the product was contaminated with this method. Lindsey's team (Rothemund-Lindsey reaction) succeeded in obtaining even higher yields, especially for tetraphenylporphyrin . Porphyrins are u. a. used in medicine, as building blocks for materials and biomedical research, as well as for enzyme models and sensors.
Individual evidence
- ^ Paul Rothemund: Formation of Porphyrins form Pyrrole and Aldehydes . In: Journal of the American Chemical Society . tape 57 , no. 10 , 1935, pp. 2010–2011 , doi : 10.1021 / ja01313a510 .
- ^ A b Paul Rothemund: A New Porphyrin Synthesis. The Synthesis of Porphin . In: Journal of the American Chemical Society . tape 58 , no. 4 , 1936, pp. 625-627 , doi : 10.1021 / ja01295a027 .
- ^ A b Chi K. Chang: Paul Rothemund and S. Ferguson MacDonald, and their Namesake Reactions - The Influence of the Fischer School on my Life in Porphyrin Chemistry . In: Israel Journal of Chemistry . tape 56 , no. 2-3 , August 2015, doi : 10.1002 / ijch.201500043 .
- ^ Paul Rothemund: Porphyrin Studies. III. The Structure of the Porphine Ring System . In: Journal of the American Chemical Society . tape 61 , no. 10 , 1939, pp. 2912-2915 , doi : 10.1021 / ja01265a096 .
- ↑ Alan D. Adler, Frederick R. Longo, John D. Finarelli, Joel Goldmacher, Jacques Assour, and Leonard Korsakoff: A simplified synthesis for meso-tetraphenylporphine . In: The Journal of Organic Chemistry . tape 32 , no. 2 , 1967, p. 476-476 , doi : 10.1021 / jo01288a053 .
- ↑ a b Jonathan S. Lindsey, Irwin C. Schreiman, Henry C. Hsu, Patrick C. Kearney, and Anne M. Marguerettaz: Rothemund and Adler-Longo reactions revisited: synthesis of tetraphenylporphyrins under equilibrium conditions . In: The Journal of Organic Chemistry . tape 52 , no. 5 , 1987, pp. 827-836 , doi : 10.1021 / jo00381a022 .
- ^ Kurt Peter C. Vollhardt, Neil E. Schore: Organic chemistry . Ed .: Eugen Müller. 5th edition. Wiley-VCH, Weinheim 2011, ISBN 978-3-527-32754-6 , doi : 10.1002 / 3527682902 .
- ↑ Joydev K. Laha, Savithri Dhanalekshmi, Masahiko Taniguchi, Arounaguiry Ambroise, and, and Jonathan S. Lindsey: A Scalable Synthesis of Meso-Substituted Dipyrromethanes . In: Organic Process Research & Development . tape 7 , no. 6 , 2003, p. 799-812 , doi : 10.1021 / op034083q .
- ↑ David Dolphin: The Porphyrins: Structure and Synthesis . No. 1 . Academic Press, Inc., New York 1978, ISBN 978-0-12-220101-1 , pp. 89-90 , doi : 10.1016 / B978-0-12-220101-1.X5001-X .