Tetraphenylporphyrin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
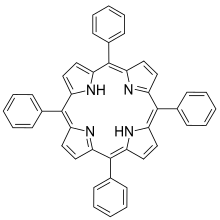
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetraphenylporphyrin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 44 H 30 N 4 | |||||||||||||||
| Brief description |
purple solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 614.74 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.244 g / cm 3 |
|||||||||||||||
| Melting point |
449-451 ° C |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetraphenylporphyrin , abbreviated TPP or 2HTPP, is a synthetic heterocyclic substance that is similar to naturally occurring porphyrins . Porphyrins are dyes and cofactors found in hemoglobin and cytochromes that are similar to chlorophyll and vitamin B 12 . Tetraphenylporphyrin is a hydrophobic purple solid that is symmetrically substituted, easy to synthesize, and soluble in non-polar organic solvents such as chloroform and benzene .
Synthesis and structure
Tetraphenylporphyrin was first synthesized by Rothemund in 1935 by allowing pyrrole to react with benzaldehyde in a bomb tube at 150 ° C. The reaction time was 24 hours and the yield was never higher than about 10%. In 1967, Adler and Longo developed a more effective synthesis by reacting pyrrole and benzaldehyde in propionic acid as a solvent . The reaction time was approx. 30 min and yields of over 20% could be achieved:
Despite the low yield, this synthesis is often carried out for teaching purposes in universities.
TPP belongs to the symmetry group D 2h . The poor symmetry is caused by the NH bonds protruding from the plane of the pyrrole rings. In contrast to natural porphyrins, H 2 TPP is in the oxidation-sensitive "meso" form and is therefore sometimes called meso-tetraphenylporphyrin. H 2 TPP can be sulfonated to obtain water-soluble derivatives :
Optical properties
Tetraphenylporphyrin has a strong absorption maximum at 419 nm (the so-called Soret band ) and four smaller maxima at 515 nm, 550 nm, 593 nm and 649 nm. It shows a red fluorescence with a maximum at 649 nm and 717 nm. The quantum yield is at 11%.
Applications
Tetraphenylporphyrin and its derivatives can act as ligands for heavy metal cations. The complexes prepared in this way have some interesting properties and can, for. B. be used in histology as a stain. Tetraphenylporphyrin itself can be used as a photosensitizer in the representation of singlet oxygen . It can also be used instead of the carcinogenic cobalt (II) chloride in combination with magnesium chloride and silica gel as a moisture indicator .
Individual evidence
- ↑ a b c data sheet meso-Tetraphenylporphine, low chlorine from AlfaAesar, accessed on May 25, 2017 ( PDF )(JavaScript required) .
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons . William Andrew, 2008, ISBN 0-8155-1990-7 , pp. 294 ( limited preview in Google Book search).
- ↑ Data sheet meso-tetraphenylporphine (PDF) from Strem, accessed on May 25, 2017.
- ↑ P. Rothemund: A New Porphyrin Synthesis. The Synthesis of Porphin. . In: J. Am. Chem. Soc. . 58, No. 4, 1936, pp. 625-627. doi : 10.1021 / ja01295a027 .
- ^ AD Adler, FR Longo, JD Finarelli, J. Goldmacher, J. Assour and L. Korsakoff: A simplified synthesis for meso-tetraphenylporphine . In: J. Org. Chem. . 32, No. 2, 1967, pp. 476-476. doi : 10.1021 / jo01288a053 .
- ↑ Falvo, RaeAnne E .; Mink, Larry M .; Marsh, Diane F .: Microscale Synthesis and 1 H NMR Analysis of Tetraphenylporphyrins . In: J. Chem. Educ. . 1999, No. 76, August, p. 237. doi : 10.1021 / ed076p237 .
- ↑ Girolami, GS; Rauchfuss, TB; Angelici, RJ, Synthesis and Technique in Inorganic Chemistry, University Science Books: Mill Valley, CA, 1999, ISBN 0-93570248-2 .
- ↑ D. Marsh, L. Mink , J. Chem. Educ. 1996, 73 , 1188.
- ↑ JB Kim, JJ Leonard and FR Longo: A mechanistic study of the synthesis and spectral properties of meso-tetraphenylporphyrin. . In: J. Am. Chem. Soc. . 94, No. 11, 1972, pp. 3986-3992. doi : 10.1021 / ja00766a056 .
- ^ Karl-Heinz Pfoertner "Photochemistry" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi : 10.1002 / 14356007.a19_573
- ↑ Yoshiyuki Fueda, Jin Matsumoto, Tsutomu Shiragami, Kazunori Nobuhara, Masahide Yasuda: Porphyrin / MgCl2 / Silica Gel Composite as a Cobalt-free Humidity Indicator . In: Chemistry Letters . tape 36 , no. 10 , 2007, p. 1246-1247 , doi : 10.1246 / cl.2007.1246 .


