Sulfenamides
| This item has been on the quality assurance side of the editorial chemistry entered. This is done in order to bring the quality of the articles on the subject of chemistry in terms of form and content to the level desired in Wikipedia. We are grateful for your help , please take part in the discussion ( new entry ) or revise the article accordingly. |
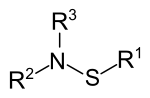
Sulfenamides belong to the group of organosulfur compounds and are the amides of sulfenic acids . The compounds are described by the structural formula R 1 S-NR 2 R 3 (R 1 , R 2 , R 3 = H, alkyl or aryl radical). Sulfenamides are used in particular in the vulcanization of rubber . The oxygen compounds of the sulfenamides are the sulfinamides (R 1 SO-NR 2 R 3 ) and sulfonamides (R 1 SO 2 -NR 2 R 3 ).
Manufacturing
Sulfenamides are usually made by reacting sulfenyl chlorides and amines :
The formation of the SN bond takes place according to the usual rules for bimolecular nucleophilic substitutions , the amine nitrogen being the nucleophilic center. Primary sulfenamides are formed by the reaction of a sulfenyl halide with ammonia . Primary, secondary and tertiary amines form sulfenamides by reaction with thiols , disulphides and sulfenyl thiocyanates. For example, triphenylmethanesulfenyl chloride and butylamine react in benzene at 25 ° C:
Numerous other reaction pathways are known. An organometallic variant uses disulfides and thiols as starting materials:
structure
The structure of the sulfenamides was characterized by means of X-ray structure analysis. The SN bond has a chiral axis that causes the formation of diastereomers . This is explained by the presence of a partial double bond, which is caused by the free electron pair on the sulfur or nitrogen atom and the occupation of an antibonding orbital on the other atom.
Space-filling substituents and the repulsion of the lone pairs of electrons further counteract the rotation around the SN bond and thus the interconversion of the diastereomers. The torsion barriers are between 12 and 20 kcal / mol.
The nitrogen atom usually forms a pyramidal structure with its bonding partners. Cyclic substituents and steric hindrance can lead to a planar structure.
Reactions
The SN bond is unstable in many ways. The sulfur atom tends to be the more electrophilic center of the SN bond. A nucleophilic attack on the sulfur can take place through amines, thiols and alkyl magnesium halides, which leads either to new sulfenamide compounds or to sulfides or disulfides.
The nitrogen and sulfur atoms of the SN bond each have free electron pairs. These lone pairs of electrons make it possible to increase the bond order to substituents or to form bonds to further substituents. For example, the nitrogen in the SN bond can be oxidized to an imine species with sodium dichromate .
Sulfenamides react with amino-azaheterocycles to form heterocyclic systems. These are used, among other things, as an amino protective group. Chlorocarbonylsulfenylchloride (ClCOSCl) form cyclic SN bonds with 2-amino-azaheterocycles.
A new variant of the Appel reaction is known for sulfenamides : reaction of o -nitrobenzenesulfonamide with PPh 3 and CCl 4 leads to o -nitro -N- (triphenylphosphoridene) -benzenesulfenamide. The triphenylphosphine forms a double bond to the sulfenamide nitrogen, contrary to the oxygen bond that is usual in the Appel reaction. In the conventional variant of the Appel reaction, the R-OH bond is cleaved and an oxygen remains on the triphenylphosphine. The SN bond is retained in the sulfenamide variant.
Applications
Sulfenamides such as cyclohexylthiophthalimide are used in large quantities in the vulcanization of rubber. The sulfenamides accelerate the vulcanization process by forming transient SN bonds. The substituents on the sulfenamide bond determine the point in time in the process at which the species becomes active. The temperature-dependent activation of the sulfenamides is essential for the vulcanization process, since the polymerization temperature determines the chain length and thus the product properties.
Individual evidence
- ↑ a b c G. Capozzi, G. Modena, L. Pasquato: Chemistry of sulphenyl halides and sulphenamides . In: Saul Patai (Ed.): Sulfenic Acids and Derivatives . John Wiley & Sons, Ltd, 1990, ISBN 978-0-470-77228-7 , pp. 403-516 , doi : 10.1002 / 9780470772287.ch10 .
- Jump up ↑ Józef Drabowicz, Piotr Kiełbasiński, Marian Mikołajczyk: Synthesis of sulphenyl halides and sulphenamides . In: Saul Patai (Ed.): Sulfenic Acids and Derivatives . John Wiley & Sons, Ltd, 1990, ISBN 978-0-470-77228-7 , pp. 221-292 , doi : 10.1002 / 9780470772287.ch6 .
- ^ IV Koval ': Synthesis and application of sulfenamides . In: Russian Chemical Reviews . tape 65 , no. 5 , 1996, pp. 421 , doi : 10.1070 / RC1996v065n05ABEH000218 .
- ↑ a b c Leslie Craine, Morton Raban: The Chemistry of Sulfenamides . In: Chemical Reviews . 89, 1989, p. 669. doi : 10.1021 / cr00094a001 .