Tacticity
The tacticity ( Greek taxis "arrangement"), also stereoregularity , describes the repeating arrangement of side chains in a polymer at certain intervals . In principle, tacticity can only occur with polymers that are built up from asymmetric monomers , e.g. B. with polypropylene or polystyrene , but not with polyethylene or polytetrafluoroethylene .
Classification
There are three basic types of tacticity:

- atactic with a random spatial arrangement of the remains.
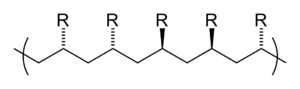
- A polymer is isotactic if all residues point in one direction.
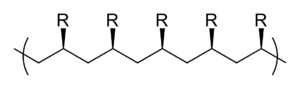
- syndiotactic , when the remnants alternately (alternately) point forward or backward.
The tacticity of a polymer can also be assessed by considering the diad sequence, i.e. H. via the spatial arrangement of two consecutive side groups. If the chain atoms of the polymer are oriented coplanar in a zigzag arrangement, an m-diad (from meso ) is obtained when two consecutive residues are on the same side of the chain. If, on the other hand, the residues are on different sides, an r-diad (of racemic ) is present. If a polymer only has m -diads - then all residues are on one side - it is again isotactic, a polymer composed entirely of r -diads is syndiotactic. In an atactic polymer, the m and r diads are found in a random order. Mesotactic polymers are a special form ; these have both tactic (syndiotactic or isotactic) and atactic sequences at the same time.
Another specialty are heterotactic polymers. These can be obtained from the polymerization of dimers (e.g. lactide , the cyclic lactone of two lactic acid molecules). The dimer already contains an m or r diad. If the polymer PLA is produced from this cyclic dimer , only every second diad is therefore determined by the stereoselectivity of the polymerization, the others are already defined in the lactide. If these diads have different orientations, a stereoregular polymer with alternating m - r - m - r - m diads, a heterotactic polymer, is obtained.
Effects
The tacticity of a polymer influences its spatial structure. The more uniform the structure, the easier it is to form a crystal structure. The degree of this crystallinity in turn influences almost all properties of the plastic, such as hardness , brittleness , dimensional stability or melting point.
In the case of some plastics, the tacticity is included in the abbreviation because of its special significance. So stands z. B. in polypropylene iPP for isotactic polymer and aPP for atactic polymer. Normal PP homopolymer (PP-H) contains not only isotactic but also atactic chain segments.
Amorphous thermoplastics like PVC and PS are usually atactic. There are also iso - (iPS) and syndio tactical (sPS) variants of polystyrene , which are capable of crystallization and are characterized by high melting temperatures, since the intermolecular interactions ( van der Waals forces ) are stronger in the densely packed crystalline areas than in the amorphous areas.
Influencing the tacticity of a polymer is by the selection of the polymerization used catalysts . When using Ziegler-Natta catalysts, largely isotactic polymers are obtained. Fully isotactic polymers are obtained through stereospecific catalysis .
Individual evidence
- ^ MD Lechner, K. Gehrke and EH Nordmeier: Makromolekulare Chemie , 4th edition, Birkhäuser Verlag, 2010, pp. 25-29, ISBN 978-3-7643-8890-4 .