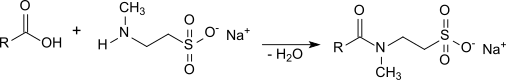Tauride
Taurides (also N -acyltaurides, N -methyl- N -acyltaurates, N -acyltaurates, or N -acyltaurines) are a group of mild anionic surfactants . Their hydrophilic head group consists of N -methyltaurine (2-methylaminoethanesulfonic acid), the lipophilic group of a long-chain carboxylic acid ( fatty acid ) linked via an amide bond . When fatty acids are lauric (C 12 ), myristic (C 14 ), palmitic (C 16 ) and stearic acid (C 18 used), however, mainly oleic acid (C 18: 1 ) and coconut fatty acid mixture (C 8 - C 18 ).
The general structural formula for Tauride is:
R is an odd-numbered alkyl radical C n H 2n + 1 with n = 7 - 17. In addition to sodium as a cation, counterions such as ammonium or other alkali or alkaline earth metals play no particular role.
History
The Tauride surfactant group, like the isethionate group , was developed by IG Farbenindustrie in the 1920s and produced under the trade name Igepon® at the Höchst plant. Because of their limescale resistance and their oil-removing effect, Igepone quickly found widespread use in textile treatment, as a detergent base and in cosmetic applications. They owe their breakthrough in particular to their property that, in contrast to soap, wool does not felt like it is washed. The production of Igepone decreased after the outbreak of the Second World War , as only inferior fatty acid qualities were available due to fat management. After the war, the Igepon trademark passed to the US company GAF (General Aniline & Film Corp., formerly American IG as a holding of IG Farben in the USA), which was taken over in 1989 by the French Rhone-Poulenc (later Rhodia, now Solvay) has been. Today Rhodia uses z. B. for Cocoyl-Tauride the trade name Geropon® TC, Clariant after taking over the specialty chemicals division of Hoechst AG in 1997 the trade name Hostapon® CT.
Manufacturing
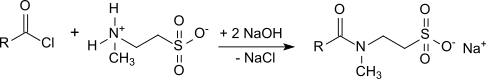
Taurides were first obtained by the Schotten-Baumann method by reacting long-chain carboxylic acid chlorides with aqueous solutions of the sodium salt of N-methyltaurine .
The occurrence of (at least) equimolar amounts of sodium chloride, which worsens the properties of surfactant mixtures with such taurides, is a problem. The high salt content also makes the taurides hygroscopic and corrosive. Another disadvantage of the Schotten-Baumann method is the necessary handling of dangerous raw materials, such as phosphorus trichloride and intermediate products, such as carboxylic acid chlorides , and the generation of large amounts of waste materials, such as phosphonic acid . This synthesis route for Tauride is therefore complex and expensive. The advantage of the Schotten-Baumann method, however, is the very low proportion of free fatty acids in the end product. Taurides can also be obtained by direct amidation of N -methyltaurine or its sodium salt with the corresponding fatty acid for 10 hours at 220 ° C. under nitrogen.
The excess fatty acid added to influence the equilibrium normally remains in the product, which can have a disruptive effect in some applications. At temperatures above 200 ° C, the decomposition of N -methyltaurine begins and the resulting taurides turn dark and have an unpleasant smell. Therefore, newer variants of direct amidation aim at gentler process management using suitable catalysts, such as. B. sodium borohydride or boric acid or zinc oxide .
properties
Taurides are mostly pasty masses at room temperature that dissolve well in water and then react neutral to slightly alkaline ( pH value 7 - 8). Their toxicity is low (7800 mg · kg −1 ( LD 50 , rat , oral , cocoyl tauride) ) They are easily biodegradable, do not tend to bioaccumulate , but - like all surfactants - are harmful to aquatic organisms. Because of their amide bond, taurides are stable in a much wider pH range (approx. 2 - 10) than corresponding esters, e.g. B. Isethionates . They are very mild surfactants with good foaming properties and high foam stability, even in the presence of fats and oils. Taurides retain their good washing properties in hard water or seawater. Because of their good compatibility with all non-ionic and anionic surfactants, taurides in concentrations of approx. 2% are ideal as so-called "co-surfactants", ie. H. surface-active substances that increase the effectiveness of other surfactants.
use
Taurides can be found as mild, well-foaming surfactants in products for body cleaning and care ( shampoos , liquid soaps and cleansers, facial tonics, skin creams, foam baths, Syndet soaps), for textile processing (wetting agents and detergents , dispersants for dyes), and in pesticides and in other industrial applications.
Trade names
Adinol®, Geropon®, Hostapon®, Metaupon®, Nikkol®, Protapon®, Pureact®, Tauranol®
literature
Wilfried Umbach (Ed.), Cosmetics and Hygiene from Head to Toe , Wiley-VCH Verlag GmbH & Co. KGaA, 3rd completely revised. u. exp. Edition (July 27, 2012), ISBN 978-3-527-30996-2 .
Individual evidence
- ^ Stefan H. Lindner: Hoechst. An IG Farben work in the Third Reich , Munich: CHBeck 2005, XVIII + 460 S., 29 figs., 20 tabs., ISBN 978-3-406-52959-7 .
- ↑ Patent US1932180 : Published on October 24, 1933 , Applicant: IG Farben AG, inventor F. Gunther et al ..
- ↑ Patent US2880219 : Published May 31, 1959 , Applicant: General Aniline & Film Corp., Inventor: LW Burnette, ME Chiddix.
- ↑ LW Burnette, ME Chiddix, Reaction of Fatty Acids with N-Methyl Taurine , J. Amer. Oil Chem. Soc., 39 (11), 1962, 477-478, doi : 10.1007 / BF02637229 .
- ↑ Patent US5434276 : Published July 18, 1995 , Applicant: Finetex, Inc., Inventor: II Walele, SA Syed.
- ↑ Patent US5496959 : Published May 5, 1996 , Applicant: Hoechst Celanese Corp., Inventor: JF Day.
- ↑ Safety data sheet for Geropon® TC 42 from Rhodia SA