Technique of positron emission tomography
The technology of positron emission tomography describes the processing steps that contribute to the creation of images in positron emission tomography and the performance parameters of a PET system.
If a positron created by the decay of the radionuclide hits an electron , both are destroyed ( annihilation ). Two photons ( gamma radiation ) are produced, which move away from each other at an angle of practically exactly 180 °. This annihilation radiation hits two detectors at the same time ( coincidentally ). As a result, the location of the positron emission can be delimited by calculation using a computer. If γ-quanta of the correct energy are detected in two detectors at the same time, this is interpreted as positron-electron annihilation at a point on the line between the two detectors (so-called Line Of Response (LOR) or coincidence line ). The technology of positron emission tomography aims to detect the highest possible rate of real such events and at the same time to keep the rate of false detections low.
principle
Similar to scintigraphy , the patient is given a radiopharmaceutical at the beginning of a PET examination , usually by injection into a vein in the arm . PET uses radionuclides that emit positrons ( β + radiation ). When a positron interacts with an electron in the body, two high-energy photons with an energy of 511 k eV each (corresponds to the frequency 123 EHz and the wavelength 2.43 pm) are emitted in opposite directions, i.e. at an angle of 180 degrees to each other ( annihilation radiation ). The PET device contains many detectors for the photons arranged in a ring around the patient . The principle of the PET examination is to record coincidences between two opposing detectors. Typical time windows for the detection electronics are 4.5 to 15 nanoseconds for this. From the temporal and spatial distribution of these registered decay events, conclusions are drawn about the spatial distribution of the radiopharmaceutical inside the body and a series of cross-sectional images is calculated. PET is frequently used for metabolic issues in oncology , neurology and cardiology .
Advantages over SPECT
With single-photon emission tomography (SPECT) a collimator is required in order to be able to determine the beam direction of the photons to be measured. Since this suppresses a large part of the photons, only about 1 in 10,000 emitted photons is detected. With PET, on the other hand, physical collimation can be dispensed with due to the measuring principle of the coincidence detection, which leads to a counting yield that is around a hundred times higher and thus improved image statistics with higher image quality and spatial resolution. The absorption of the measured photons depends only on the thickness of the irradiated tissue, but not on the location of the photons (see Correction of the measurement data, section absorption correction ). This enables an exact quantification of the tracer distribution in the examination volume, which is not possible with SPECT.
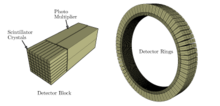
PET detector
With a discrete 511 keV, the energy of the destruction radiation to be detected is greater than the maximum energy of the X-ray spectrum used in X-ray diagnostics (up to 150 keV in computer tomography). The probability of interaction with matter is therefore comparatively low. This property, which is positive from the point of view of radiation protection, makes detection and thus imaging more difficult. Detectors for PET could also be implemented using semiconductor technology, but a combination of scintillation crystal and photomultiplier is currently used in all clinical PET .
The ideal PET detector
There is no such thing as the ideal PET detector. The following properties are desirable:
- It completely encloses the examination subject.
- It completely absorbs all incident photons.
- It registers the exact position of the incident photons.
- After detecting a coincidence event, it is able to register the next event again very quickly (short dead time ) and thus does not lose its performance even at the highest counting rates.
- The detection electronics of the detector determine the time of the annihilation so precisely that it can determine the exact location of the annihilation via the time-of-flight difference of the annihilation photons.
- The coincidence time window is so small that random coincidences (= random) play no role.
- The detector can determine the direction of incidence of the photons; Randoms are thus greatly reduced, since the number of possible detectors for the corresponding second photon is severely restricted.
There is no ideal material for the scintillator of the PET detector. The following properties are desirable:
- High linear attenuation coefficient µ for high sensitivity .
- High photo fraction , since only photoelectrons but not Compton photons are detected.
- Short scintillation decay time for small numbers of random coincidences at high count rates.
- Bright scintillation for good energy resolution in the photomultiplier.
- Good energy resolution of the detector material for clear differentiation from scattered radiation.
- Scintillation wavelength close to 400 nm for good detectability in the photomultipliers .
- Transparent for photons of the scintillation wavelength.
- Refractive index close to 1.5 for good light transfer from the crystal to the photomultiplier.
- Radiation-proof for stable performance at high dose levels.
- Not hygroscopic for easy packing.
- Inexpensive to manufacture.
- Robust for easy handling and smaller crystal dimensions.
- Consistent sensitivity for low recalibration effort .
The real PET detector
geometry
In the early days of PET systems were prepared in which the coincidences between partial rings or between the heads of a connected in coincidence dual head gamma camera with NaI were measured scintillator. However, the sensitivity of these systems was so inferior to full ring PET systems that they could not prevail.
The first full-ring PET had only one detector ring, today several rings are arranged next to each other in modern systems, which increases the sensitivity of the overall system.
The detector of a PET scanner available today consists of several detector rings, each made up of 30-40 detector modules. A detector module consists of 4–8 detector blocks. A detector block consists of several individual crystals (e.g. in the arrangement 4 × 4, 4 × 5 or 6 × 6). The dimensions of the crystals range from 6 to 8 mm in the transaxial direction. In the radial direction - i.e. in the direction of incidence of the photons - the crystal thickness is usually between 20 and 30 mm. In total this results in approx. 10,000 ring-shaped detector crystals ( scintillation counters ), which are coupled with approx. 1000 photomultipliers .
The axial field of view of the detectors - also called Field of View ( FOV ) - is in the range of approx. 15 to 20 cm. The diameter of the detector ring is between approx. 50 and approx. 85 cm, depending on the device.
Crystal material
All systems for clinical PET today use either bismuth germanate "BGO" (Bi 4 Ge 3 O 12 ), compounds doped with Ce 3+ such as lutetium yttrium oxoorthosilicate ("LYSO", LuYSiO 5 : Ce 3+ ) or lutetium oxyorthosilicate ("LSO" ) as detector material , Lu 2 SiO 5 : Ce 3+ ).
The shorter decay time of LSO and LYSO allows PET systems to be built with significantly smaller coincidence time windows than is possible with the crystal material BGO. A smaller coincidence time window reduces the number of measured random coincidences and in this way improves the signal-to-noise ratio. However, LSO and LYSO are (currently) significantly more expensive to manufacture than BGO. The sensitivity and photo fraction of BGO are greater than those of LSO and LYSO. Building a time-of-flight PET system, which has been researched since the 1980s, is not possible with BGO. The time resolution required for the TOF cannot be achieved with this. All commercially available TOF PET systems use LSO or LYSO as the crystal material. Until a few years ago, a manufacturer also used gadolinium orthosilicate (GSO) as a detector material.
Scintillation detection
The scintillation crystals are either sawn in or several crystals are glued together. At the crystal boundaries, the photons are reflected, which are thus directed towards the photomultiplier . This enables a more precise localization than in a homogeneous, single crystal block. Photomultipliers are used in all clinical PET systems because they are (currently) the most sensitive detection instruments for the very weak flashes of light. Several photomultipliers “look” together at a group of scintillation crystals and are connected to the scintillation crystal either directly or via light guides. The localization of the scintillations takes place according to the principle of the Anger camera by weighting the brightness of the scintillations registered in the photomultipliers. The photomultiplier have round or square entry windows depending on the manufacturer.
Desire and reality: what do you want to measure and what is measured?
Coincidence radiation can be scattered and absorbed on the way into the detectors. No detector has one hundred percent detection sensitivity. Detectors require time for the measurement and even the smallest time window is no guarantee that only coincident events will be recorded.
The following describes the effects that occur when counting and localizing the coincidence radiation and which recording, correction and reconstruction processes are used to try to keep influences that impair the image quality to a minimum:
True coincidences ("Trues")
The aim of PET is to measure only “trues”. A true is when two photons produced were able to cross the examination volume without interaction (scattering) and have deposited their full energy in the detectors, which were then also recognized by the measuring electronics. The requirements that a True can be measured are:
- The direction of flight of both photons is in the field of vision of the detectors.
- Neither of the photons has lost too much energy through scattering (in the patient), so that both are detected.
- None of the photons disappeared due to absorption.
- The system's detectors are sensitive enough to detect them.
- The detectors of the system are not blocked by previous events at the time of the scintillation (so-called dead time ).
It is clear that the goal of good device design must be to get a high number of trues . The higher the number of trues for a certain activity, the more sensitive the PET is.
The number of Trues can be increased by:
- Increase in the applied nuclide activity: However, this also increases the number of singles and thus also the random .
- Small patient diameter, which also reduces the scattering of photons ( scatter )
- Increase the recording time
- large solid angle covered by the detector
- The detectors have a narrow measuring range around the expected energy
- high detection sensitivity of the detector crystal
- high detection sensitivity of the detector electronics
With an increasing counting rate, the dead time of the detector crystal and detector electronics becomes more important, since with increasing activity, the probability increases that a registered coincidence event will be followed immediately by another.
Single events ( singles )
Singles are one of the adverse events. They arise when only one of the two produced photons can be detected. The reason for the loss of detection of the second photon can be:
- One of the two photons leaves the "field of view" of the detectors (Field-of-View, FOV)
- One of the two photons is scattered in the examination volume (patient). The associated change of direction may lead to the photon leaving the FOV of the detector. However, a photon loses energy due to scattering: if it still hits a detector after scattering, it is discarded when its residual energy falls below the lower energy threshold of the detector.
- One of the two photons is absorbed in the examination volume (patient).
- One of the two photons can penetrate the detector or only deposits part of its energy in the detector; in this case the photon is interpreted as a scattering photon and is rejected.
- One of the two photons hits a detector that is busy processing a previous pulse. The measuring electronics are not able to record two simultaneous or almost simultaneous impulses and reject the second or both scintillations (= dead time of the measuring system).
From the description of the causes of singles it becomes clear that there are only limited possibilities to minimize their number. These are:
- Large field-of-view or large number of detectors switched in coincidence and thus high spatial coverage.
- Use of fast detection electronics that show only minor dead-time effects.
- Use of a detector with high sensitivity that "overlooks" only a few photons. The decisive factors are the detector material and the thickness of the scintillation crystal. The detectors of clinical PET / CT systems can (depending on the system) detect approx. 80 to 95 percent of all incident photons.
If a single is recognized as such, it is discarded and does not contribute to the creation of the image.
Random Coincidences ( Randoms )
Randoms are an adverse event. If two scintillations are detected in two coincident crystals within the coincidence time window, they are interpreted as annihilation.
However, it is possible that two singles are registered that happened to be created at different locations in the investigation volume at the same time. This is then also - in this case incorrectly - interpreted as an annihilation and is included in the image reconstruction.
The probability of occurrence of random coincidences ( Randoms ) can be reduced by:
- a low applied nuclide dose,
- a small coincidence time window,
- a large number of detectors,
- by reducing the number of measured singles (e.g. by using septa = 2D recording mode)
- by using the "time-of-flight" data acquisition technology
The number of measured random rises sharply with the number of singles.
Scattered coincidences ( Scatter )
Scattered radiation is one of the undesirable events. If a photon is scattered on the way to the detector, it changes its direction. However, since the location determination in the PET is always based on a straight route between two events occurring at the same time, this leads to incorrect localization. However, due to the scattering, a photon loses energy. Scattered radiation can thus be masked out in that a scintillation is only counted if it exceeds a certain energy threshold in the detector. Using a lower energy threshold is therefore an effective method of suppressing scattered coincidences. However, this approach is limited by the finite energy resolution of the detector.
However, scattered radiation can also be reduced by using partitions (“septa”) or endshields; in this case, scattered photons that do not come from the measurement volume do not even reach the detector.
The transition from 2D to 3D acquisitions in clinical practice and the associated elimination of septa are associated with a greatly increased proportion of scattered radiation. For this reason, there are various approaches to eliminate the scattered radiation component with the help of correction algorithms.
Acquisition modes
As already described, one is confronted with some undesirable side effects when recording the coincidence events. In some cases, contradicting performance parameters are required of the detector system. In addition, the question to be clarified determines how the measurement data is recorded or post-processed. Special acquisition modes have been developed in order to achieve optimal results for the respective application.
Static data acquisition
The most commonly used recording mode is static recording. All events that occur at the same recording position during a certain period of time are used for the image reconstruction. Typically, for an FDG-PET, coincidences are acquired over a period of two to four minutes for each recording position. The longer the recording runs, the greater the number of coincidence events that can be used for image reconstruction, which improves the image quality in terms of the signal-to-noise ratio. On the other hand, however, lengthening the recording time increases the probability of movement artifacts due to voluntary and physiological movements of the examination subject. A static exposure provides information about the amount of tracer accumulated in the examination volume at the time of exposure. The rate of enrichment cannot be assessed with this, dynamic acquisition is required for this.
Dynamic data acquisition
Unlike the static recording, the total number of coincidence events is not added, but the course of the activity enrichment is considered. The speed, i.e. H. The dynamics of the enrichment allow conclusions to be drawn about the type or severity of a lesion . The dynamic admission procedure takes place u. a. Use in receptor scintigraphy (neurology) or for assessing myocardial perfusion . A dynamic study consists of multiframe data sets. In contrast to the static recording, an image sequence in a multiframe data record does not show a sequence of different recording positions, but different time windows of the same recording position, e.g. B. Frame 1: 0-15 seconds, Frame 2: 15-30 seconds, etc.
Triggered data acquisition
It is a special form of dynamic data acquisition. Breathing and heartbeat are patient movements that cannot be suppressed during the PET scan. It is therefore advisable to eliminate the associated image artifacts by means of suitable data acquisition. With triggered data acquisition, a complete cycle (heartbeat or breathing ) is recorded by a measuring system and the recorded raw data is divided into (e.g. 8 to 16) groups (so-called gates or bins). After the exposure, these are reconstructed in a summarized manner. The results are images that show only one phase of movement (end- systolic or end-diastolic phase with ECG triggering, end-inspiratory or end-expiratory phase with breath triggering) and show no blurring artifacts. Clinical studies prove the diagnostic added value of the procedure. The ability to assess the dignity of pulmonary nodules has been proven to improve through the use of breath triggering, the quality of cardiological recordings through the combined ECG and breath triggering.
The 3D recording mode
Here, a check is not only made for coincidences within the same detector ring, but also between different detector rings. The 3D recording mode is by far the most frequently used recording mode in today's devices (as of 2010). It is also the only acquisition mode available for a large number of clinical PET and PET / CT systems.
Since events from a larger number of detectors are viewed in the 3D recording mode, this places greater demands on the detector and device electronics, which must be able to register a multiple number of events. The pure 3D mode also leads to an inhomogeneous sensitivity in the axial direction: In the center of the axial FOV, the solid angle of the detectable coincidence events is larger than at the edge, where almost only strictly radial coincidences can be measured. Since a coincidence connection of all detectors to all detectors cannot be implemented anyway, this effect is reduced by skillful grouping of the interconnected detectors, but cannot be completely eliminated.
In the 3D recording mode, the detectors are exposed to strong scattered radiation, which deteriorates the image quality with increasing examination volume and increasing dose rate. The proportion of scattered radiation increases u. a. because the path length of a photon - and thus the probability of scattering / absorption of one of the two photons - is greater with an oblique passage through the examination volume than with a strictly radial passage (here radial: towards the detector, axial: from the examination volume towards the head / Feet of the patient).
The gross counting rate in 3D mode is a factor of 8 higher than in 2D mode, since inclined coincidences are also recorded. At the same time, however, the proportion of scattered radiation increases by a factor of 3 from approx. 10% to 35 to 45%. The net gain in real measured coincidences is therefore only about a factor of 5. In the slices at the axial end of the detector, in the absence of corresponding inclined coincidence lines, measurements are ultimately made in 2D mode, although the scattered radiation is due to the lack of septa is not hidden. The signal-to-noise ratio is therefore even significantly worse there than in the 2D recording mode. This is another reason why recordings in 3D mode have to be made with a significantly higher layer overlap (25 to 40%) than in 2D mode (approx. 2%).
The greatly increased proportion of scattered radiation also results in significantly higher requirements for the scattered radiation correction, detector normalization and attenuation correction algorithm used in the image reconstruction. Especially in the early days of the 3D scanner, this, like the considerably larger raw data sets, led to sometimes drastically longer image reconstruction times (30 minutes and longer) compared to 2D mode. However, this problem has been eliminated in recent years due to the availability of correspondingly powerful computer hardware.
The 2D recording mode
In addition to the 3D mode, a few devices still have a 2D recording mode today. Only those events are checked for coincidence that occur in crystals of the same detector ring. A further distinction is made between an “electronic” and a “real” 2D mode: In the “real” 2D recording mode, tungsten septa are moved into the gantry in order to stop photons that are not strictly radial in origin. In the "electronic" 2D mode, the coincidence check is only checked for coincidences within the same ring, but the radiation itself remains "visible" to the detectors. The electronic and the "real" 2D mode lead to first-class homogeneity of the sensitivity across the field of view.
The physical masking of obliquely arriving photons in the “real” 2D recording mode using tungsten septa and endshields greatly reduces the number of events “seen” by the detectors; this recording mode can therefore be used very effectively for suppressing scattered radiation. Since the detectors only “see” coincidences from a radial direction, the probability that measured events are random coincidences is also lower than in the 3D recording mode. By blanking out inclined events, not only undesired scattering events, but also real coincidences are naturally kept away from the detectors. The sensitivity of the scanner in 2D mode is only about 20% of that in 3D mode, but the proportion of scattered radiation also drops by over. 40% to approx. 10%.
Before the appearance of LSO-based PET systems, all PET scanners were 2D scanners. PET systems with "fast" crystals (LSO / LYSO) usually do not have the "real" 2D recording mode. The manufacturer argued that the significantly smaller coincidence time window of around 5 ns compared to around 10 ns for BGO scanners effectively suppresses the occurrence of random coincidences.
The 2D recording mode was used when the examination volume is large and a large number of scattering events could be expected, and the injected nuclide dose was high, which also increased the occurrence of random coincidences. In the latter case, the loss of sensitivity is not very serious, since the counting statistics for these recordings are very good anyway and the advantage of scattered radiation suppression considerably exceeds the loss of sensitivity . Since the counting rate (NECR) to be processed drops drastically through the use of septa, this mode is also ideally suited for imaging very short-lived nuclides, which require the measurement electronics to process the highest nuclide activities.
The devices of the BGO-based DST-E series from General Electric were the last clinical systems that still had a real 2D mode. The BGO-based successor Discovery 600, which was presented at EANM in autumn 2008, only has the 3D recording mode. The manufacturer is of the opinion that the effects of undesired scattered radiation can be effectively suppressed with the help of more powerful electronics and its iterative reconstruction mechanism.
Time of Flight
A TOF measurement measures the time difference between the impact of the two gamma quanta within a coincidence window of around 6 ns. This not only makes it possible to make a statement about the course of the line of response, but also to determine the position of the annihilation that has taken place on this line. With a temporal resolution of 600 picoseconds , the location of the annihilation can be determined with an accuracy of 9 cm FWHM . With these parameters, the limit with the best possible device design, small lesion size and a patient diameter of 40 cm is 50% gain in signal-to-noise ratio. The TOF measurement therefore improves the signal-to-noise ratio and thus the achievable spatial resolution . In contrast to the real 2D recording mode, this gain in signal-to-noise ratio occurs without loss of sensitivity. A further improvement in the temporal resolution will simplify the currently very complex analytical and iterative image reconstruction methods.
Correction of the measurement data
Due to the influences mentioned, the measured data are subject to various types of errors and for this reason must be corrected several times before the image reconstruction.
Absorption correction
While an absorption correction of emission data with gamma cameras has not yet achieved the acceptance it is due, with PET it is an indispensable necessity for image reconstruction and the quantification of enrichments, because the radiation is weakened by one to two orders of magnitude when passing through the examination object. The absorption correction is the largest amount of all corrections that are used in the image reconstruction of PET data.
Explanation:
Imagine a nuclide accumulation that is located near a water-filled cylinder with a diameter of 30 cm. The nuclides that decay there emit their annihilating radiation in all spatial directions. Two cases are to be considered: Case 1: The two resulting photons 1 and 2 fly exactly tangential away from the object surface (violet arrows), neither photon 1 nor photon 2 penetrates any part of the cylinder. Case 2: Photon 1 aims radially towards the center of the cylinder. Naturally, photon 2 moves away from the cylinder without penetrating even part of the cylinder (red arrow pointing upwards). The probability is high that photon 1 absorbs water when passing 30 cm and thus photon 2 becomes a “single” and is therefore no longer available for image reconstruction.
If the absorption of the radiation were not taken into account in the image reconstruction, artifacts would be the result; the measured activity distribution did not agree with the actual distribution without absorption correction.
Relevance for the quantification of enrichments:
The probability of an absorption in case 2 (red arrows) is independent of whether the annihilation took place on the surface (location A) or in the middle of the object (location B). With annihilation on the surface (location A), photon 1 passes through the entire cylinder, photon 2 only air, with annihilation in the middle of the cylinder (location B), both photons pass through half the cylinder, which leads to the same absorption probability. Since the absorption probability only depends on the overall attenuation of the irradiated volume, but not on the location of the annihilation on the coincidence line, this enables the depth-independent quantification of the nuclide enrichment.
Absorption correction for PET:
In the case of the pure PET systems built up to around 2003, the emission data of the PET was corrected for attenuation with the help of rod sources. For this purpose, these were guided around the examination subject and a tomogram was created similar to that used in computer tomography. This was an expensive and time consuming process. In addition to the emission measurement, an approx. Three-minute transmission measurement was necessary for each bed position. The 68 Ge source used for this disintegrated, which over time not only worsened the quality of the attenuation correction, but also represented a constant cost factor.
Absorption correction in PET / CT:
In today's PET / CT systems, the attenuation correction is carried out on the basis of the CT data. Since a full-body image with a modern CT does not take longer than 30 seconds, this procedure is not only much more precise, but also considerably faster. With the help of conversion tables, a Hounsfield value in the computed tomography section is assigned the associated linear attenuation coefficient µ for gamma radiation with an energy of 511 keV. Before this, however, the CT data are segmented: the measured data are smoothed and the Hounsfield values rounded up or down to a fixed attenuation value for water, bones and air. This prevents image noise from being added to the image as a result of the attenuation correction. Since the computed tomogram is often recorded using X-ray contrast media, this can lead to image artifacts in some devices. A metal implant can also interfere not only with the image reconstruction of the CT, but also the attenuation correction and thus the image reconstruction of the PET image.
Correction of the Randoms
As described , the number of random coincidences depends on various parameters and can assume very high values; therefore, the random rate must be subtracted from the measured count rate. There are two different ways to measure the rate of random coincidences:
- Either you measure the number of singles and use their rate to calculate the expected number of randoms, or
- after a coincidence measurement that contains real (trues) and random (randoms), a second, shifted, equally large time window that cannot contain real coincidences is measured. Since the measurement in this second time window was not triggered by a real coincidence event, the coincidences determined there must be random coincidences.
Dead time correction
As the count rate increases, the dead time of the measuring system becomes more important. At very high counting rates, the measured counting rate deviates so much from the real counting rate that this deviation has to be corrected if the correctness of the measurement is to be obtained. The implementation is simple: You create a calibration series with increasing activity. This known activity is measured in diluted form with a reference measuring device (e.g. borehole) and a correction factor valid for the respective activity is calculated.
Correction of scattered
radiation Scattered radiation occurs in the vicinity of major activities or in the vicinity of objects with strong attenuation. A correction function can either be determined using phantom measurements or calculated using attenuation data.
Recovery correction
The spatial resolution of the system also determines the measured activity of a lesion. Without this correction, lesions smaller than four times the spatial resolution of the system are displayed with reduced uptake. The deviation is corrected with the aid of a recovery coefficient (Hot Spot Recovery Coefficient HSRC and Cold Spot Recovery Coefficient CSRC). The method can be used with good results up to a lesion size which corresponds to 1.5 times the resolution of the system. In the case of lesions that are smaller, statistical errors are too large due to the greatly increased noise component. The Hot Spot Recovery Coefficient can also be used as a test for system linearity.
Image reconstruction


The image reconstruction creates the image from the multiple corrected measurement data, which is the basis for analysis and diagnosis.
Filtered back projection
The filtered back projection (also FBP for filtered back projection ) is a procedure that is primarily used today in computer tomography . In PET it has meanwhile been supplanted by the iterative reconstruction method.
Iterative 2D reconstruction
As in mathematics , this is a method in which a solution is gradually approached by repeatedly applying the same algorithm (from the Latin iter “step”). The 2D methods are called MLEM , OSEM or AW-OSEM .
All of these methods start with an assumed tracer distribution, which is approximated to the actual conditions by comparison and correction with each calculation run. The approximation steps are:
- Back projection of the assumed tracer distribution taking into account the properties of the imaging system: What would be measured if the tracer were distributed as assumed in the model
- Determination of the difference between back projected and measured data
- Calculation and application of the correction factor obtained from the difference
- Repeat the previous steps until a termination criterion is reached.
If the data to be reconstructed was measured with a 3D acquisition, it is converted beforehand by Fourier rebinning so that it can be reconstructed with the aid of the aforementioned 2D method.
What all iterative methods have in common is that they are very computationally intensive. In principle, any resolution can be achieved with an iterative reconstruction process, but the image noise is often increased and rounding errors have an increasing effect, so that further iterations then worsen the image quality.
Iterative 3D reconstruction
Iterative 3D processes have not been around for very long. Even the 2D method requires around ten times as much computing power as the filtered rear projection and has only been used for a few years due to this fact.
Iterative 3D methods are mathematically very demanding, although the basic principle is the same as that of 2D reconstruction. In 2007, the 3D iteration processes RAMLA (Philips) and Vuepoint (General Electric) were used in PET.
At the SNM 2007, Vue Point High Definition from General Electric and Truepoint HD (Siemens) presented new iterative 3D reconstruction methods. Vue Point High Definition improves the signal-to-noise ratio by approx. 60 percent and enables resolutions that are below 3.5 mm under clinical conditions. The special feature of the reconstruction is that it processes all corrections in the iterative loop and thus solves convergence problems of previous iteration methods.
Truepoint HD reconstruction (Siemens) is based on the point spread function. The imaging properties of the detector are modeled and corrected. Siemens states that it can achieve resolutions of up to 2 mm under laboratory conditions with Truepoint HD.
The use of an iterative image reconstruction algorithm enables the imaging properties of the system to be taken into account in the image reconstruction. In this way, the proportion of scattered radiation can be reduced, which improves the signal-to-noise ratio.
Performance parameters of a PET system
Critical performance parameters are always determined by the question. When searching for metastases, the PET must be able to display increased uptake in both activity-poor and activity-rich surroundings. In nuclear cardiology, on the other hand, the representation of a reduced uptake in an active environment is important. In general, the following applies: Small and large areas with different tracer distribution from the environment must be correctly identified and quantified by the PET. The following performance parameters describe how well a system can meet these requirements.
sensitivity
The sensitivity of a PET scanner is its most important device property, as it determines the image quality and recording time. In general, sensitivity is the total number of true positive events in relation to the sum of true positive and false negative events. In PET, sensitivity is the number of pulses per second (trues) that are measured per becquerel and milliliter . It is usually given in. When measuring the activity of an injected sample, a logging station is used that is believed to be able to detect any scintillation . Good counting rate statistics are a basic requirement for the reconstruction of a good quality image; it can only be achieved if the PET can determine as many trues as possible from the injected dose . Good systems achieve values from 7 to 9 . With PET, the sensitivity of the system depends on the photo fraction and the absorption coefficient of the detector material, on the detector geometry and the crystal thickness. The sensitivity of a system can be significantly improved by increasing the axial FOV (adding an additional detector ring). However, since this also records coincidences that run obliquely through the examination volume, the probability increases that these photons are scattered, which is associated with a loss of image contrast from regions of low enrichment.
Scattered radiation fraction
This is the proportion of scattered and random coincidences in the total number of measured coincidences. The lower the amount of scattered radiation, the better the image contrast. The thickness of the irradiated volume essentially determines the proportion of scattered radiation. Images of obese patients are therefore noisier than scans of lean people.
The amount of scattered radiation can be reduced by:
- Use of the time-of-flight technique
- Application of a powerful iterative image reconstruction
- Shielding of scattered radiation by septa and endshields; these are panels attached to the front of the detector; Disadvantage: septa reduce the sensitivity of the system
- a small coincidence time window
- a good energy resolution of the detector, which enables a good differentiation between scattered and non-scattered photons (scattered photons have an energy <511 keV).
Spatial resolution
The spatial resolution that can be achieved by the PET system is specified in FWHM . It is limited by the following factors:
Size of the scintillation
crystals : The smaller the area of the individual crystals facing the measurement volume, the better the resolution of the detector system. However, smaller crystals reduce the sensitivity and a larger number of detectors increases the system costs.
Collinearity error:
The emission of the photons is not exactly collinear, but with a small deviation from the ideal 180 degree angle. The reason is that the positronium moved before the annihilation . Since the angle of incidence of the photons cannot usually be measured during the reconstruction, a straight line must be assumed as the line-of-response (LOR) for the reconstruction, which results in an error of a few millimeters in whole-body PETs. This so-called collinearity error can be avoided. Detectors with measurement of the entrance angle for clinical PET systems are in the development phase and are already commercially available for preclinical PET systems for animal experiments. So-called Phoswich detectors are used to correct the error, in which two different detector materials are arranged one on top of the other in a quasi sandwich construction.
Gantry diameter:
A larger gantry diameter increases the influence of the collinearity error of the positron radiation and thus reduces the maximum achievable resolution. A small gantry diameter increases the resolution that can be achieved in the center of the FOV, but leads to a disproportionately strong decrease in the spatial resolution outside the center of the image: Coincidences that take place outside the center of the FOV enter the detectors more obliquely the further they are in the radial direction away from the center of the gantry. The detector then does not see a compact flash of light coming from the front, but rather a light trail, the exact location of which cannot be determined.
Mean free path:
Immediately after their formation, the positrons are too fast to annihilate with an electron. They therefore move a short distance from the place of their origin, whereby they continuously lose energy through interaction with other particles. The distance covered up to the annihilation depends on the mean density of matter in the environment and the initial energy of the positrons - and thus on the radionuclide used. This so-called mean free path is of the order of 0.5 millimeters in the tissue and 1.5 millimeters in the lungs.
Localization accuracy of the scintillation:
The detector size and the localization accuracy of the scintillation site with the help of the Anger principle limit the localization accuracy of a scintillation in the crystal to approx. Two millimeters.
Smoothing
filter : The smoothing filter used in the image reconstruction algorithm to reduce image noise reduces the spatial resolution by approx. 2–5 millimeters
Presentation matrix:
The presentation matrix used, i.e. H. Pixel size of the image, reduces the resolution of the image.
Physiological patient movement:
The patient movement that may a. caused by the breathing movement of the patient leads to a smearing of the image information of up to 50 mm.
The numerical values of the uncertainties mentioned do not simply add up; they must be superimposed according to the rules of error propagation . Disregarding patient movement, a system resolution between 3 and 6 millimeters can be achieved in the center of the image.
Noise Equivalent Count Rate, NECR
If you measure very little activity on the PET in a test series and increase it slowly, the following picture emerges:
The number of measured true coincidences initially increases with the dose. Random coincidences, however, increase more than linearly (generally quadratically) with increasing activity, and at some point their number even becomes larger than the number of true coincidences (trues), as the probability increases that two random events occur within the coincidence time window.
In addition, dead-time effects become noticeable, since the crystal and electronics require a fixed period of time to detect a scintillation. If the next event takes place during this period, it cannot be recorded and is discarded.
For the image quality, this means: If the activity to be measured is very small, the image is very noisy because the number of events measured is low. It improves with increased activity, but eventually reaches a maximum. A further increase in dose leads to a significant loss of image contrast, and the image becomes flatter again.
The NECR (= Noise Equivalent Count Rate) describes this property of a PET: with = rate of true coincidences = rate of scattered coincidences = rate of random coincidences = area proportion of the projected object on the projection surface
A good PET system has a high maximum NECR at a clinically achievable concentration of activity.
The shape of the NECR curve depends heavily on the device design and the examination subject. The diagrams shown by the manufacturers were measured in a standardized NEMA phantom. However, if the examination object is larger than the NEMA phantom, for example, the NECR curve reaches its maximum earlier, because the measured proportion of scattered radiation increases significantly (corpulent patient).
Axial field of view ( axial field of view , AFOV)
The total recording time depends on the recording time per bed position and the number of bed positions that are required to image an examination subject. Today's PET scanners have several rings and thus a field of view of 15 to 25 cm. In addition to the size of the axial FOV, the so-called “slice overlap” also plays a role. Since the sensitivity of the detector decreases towards the edge of its axial field of view, the recording must be overlapping: When recording the next bed position, a small area is recorded again that was already displayed in the previous bed position. Due to their very homogeneous sensitivity in the axial direction, real 2D scanners only require an overlap of one to three percent, 3D scanners require an overlap of 20 to 40 percent, which leads to a significant increase in the examination time and the effect of increasing the sensitivity of the 3D mode partially picks up again. A PET scanner that captures the entire patient with a very large axial field of view using a single image would be ideal. Such a device design has not yet been implemented, among other things for cost reasons.
Individual evidence
- ↑ Bernd J. Krause, Andreas K. Buck, Markus Schwaiger: Nuclear medicine oncology. ecomed Medicine, 2007, ISBN 978-3-609-76308-8 , p. 20.
- ↑ a b L. Geworski: Online requirements for quantification in emission tomography. Habilitation thesis, Humboldt University Berlin, 2003
- ↑ Charles L. Melcher, Scintillation Crystals for PET, J Nucl Med 2000; 41: 1051-1055 ( Memento from September 13, 2006 in the Internet Archive ) (PDF; 59 kB)
- ↑ a b c d e R. Standke: Technical basics of 18 F-fluorodeoxyglucose-positron-emission-tomography-diagnostics ; Acta Medica Austriaca, Blackwell Verlag, Volume 29, Issue 5 2002, pp. 149–155. doi: 10.1046 / j.1563-2571.2002.02040.x
- ↑ a b c d Werling, Alexander, Model-based correction of scattered radiation in positron emission tomography
- ↑ Helmholtz-Zentrum Dresden-Rossendorf: Correction of movement-related artifacts in whole-body examinations ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ^ A. Martínez-Möller, W. Howe, M. Schwaiger, S. Nekolla Motion Free Images by Dual Gating of PET Listmode Acquisitions World Congress of Cardiology, held in Barcelona, Spain, September 2006
- ↑ 4-D PET / CT Keeps Clinicians on Track, article on http://new.reillycomm.com/ ( Memento of April 13, 2008 in the Internet Archive )
- ^ Cyrill Burger, David Townsend: Basics of PET Scanning, from: Gustav K. von Schulthess: Molecular Anatomic Imaging, PET-CT and SPECT-CT integrated modality imaging, Lippincott Williams & Wilkins 2007
- ↑ Discovery 600 product information on www.gehealthcare.com ( Memento of the original from April 9, 2011 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Article by Michael Haas on Imaging Technology News ( Memento from April 13, 2008 in the Internet Archive )
- ↑ S. Surti, S. Karp, LM Popescu, E. Daube-Witherspoon, M. Werner; Nat. Institutes of Health, Philadelphia, PA, USA: Investigation of time-of-flight benefit for fully 3-DPET. In: IEEE Transactions on Medical Imaging Vol. 25, No. 5. IEEE Engineering in Medicine and Biology Society, May 2006, pp. 529-538 , accessed August 22, 2010 .
- ↑ Panin et al. (Siemens) “Fully 3-D PET Reconstruction With System Matrix Derived From Point Source Measurements”, IEEE Medical Imaging, Vol. 25, no. July 7, 2006.
- ↑ European Journal of Nuclear Medicine, Vol 30, No. November 11, 2003
- ↑ A Comparison of the Imaging Properties of a 3- and 4-ring Biograph PET Scanner Using a Novel Extended NEMA Phantom, C. Jonsson, Member, IEEE, R. Odh, PO. Schnell and SA Larsson, Member, IEEE, 2007 IEEE Nuclear Science Symposium Conference Record, M13-25
- ↑ Sánchez-Crespo, et al. “Positron flight in human tissues and its influence on PET image spatial resolution”, Eur J Nucl Med, Vol 31, Iss 1, Jan 2004, pp 44-51.
- ^ A b Charles Stearns, Alexander Tokman: Design Criteria for PET Scanners: What is important and Why; Contribution from: Gustav K. von Schulthess: Molecular Anatomic Imaging, PET-CT and SPECT-CT integrated modality imaging, Lippincott Williams & Wilkins 2007