Thermal layers
Thermal layers represent layers of water at different temperatures in the body of water. Due to the density anomaly of the water, they occur in deeper, reasonably calm bodies of water (see properties of water ).
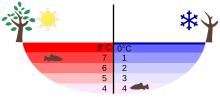
Fresh water has the greatest density at a temperature of 4 ° C (exactly: 3.983 ° C) . If it is heated or cooled, the density drops. The densest water sinks to the bottom of the water. Above that there is lighter or less dense water. However, since both warmer and colder water are lighter, a layer with, for example, slightly warmer water forms first. Above it is a layer of the same density with slightly colder water. A reverse shift sequence is also possible.
This stratification continues up to a water temperature of around 8 ° C. At this temperature the density is equivalent to the density of water at 0 ° C. Below this equivalent , ice would form, which however always has a lower density than water.
Thermal layers can only form in deep, sufficiently calm waters. The water mixes in shallow or choppy waters.
Thermal layers are of particular importance in sonar . The different physical properties create an impedance contrast so that the individual layers partly reflect the sound . Submarines can use this effect to hide from a warship , for example .