U0126
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
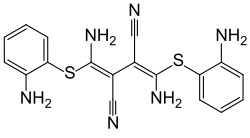
|
||||||||||
| General | ||||||||||
| Surname | U0126 | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 18 H 16 N 6 S 2 | |||||||||
| Brief description |
|
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 380.49 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
172-173 ° C (monoethanolate) |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
U0126 is a selective inhibitor of the enzymes MEK 1 and 2 ( mitogen-activated protein kinase kinase family members ) in the MAP kinase pathway .
properties
When inhibited with U0126, the transcription factor AP-1 becomes non-competitive with an IC 50 of 72 nM for MEK1 and 58 nM for MEK2. The mTOR -p70 (S6K) path is also inhibited. U0126 shows anti- tumor effects and intensifies an anoikis in cell cultures . U0126 is also an inhibitor of PKC , Raf , JNK , MEKK, MKK-3, MKK-4 / SEK, MKK-6, Cdk2 and Cdk4 . U0126 activates the aryl hydrocarbon receptor and gene expression of CYP1A and CYP3A .
Applications
A use of U0126 for the treatment of post-traumatic stress disorder is being investigated. U0126 protects nerve cells from oxidative stress in cell cultures. U0126 slows down Waller's degeneration after an injury to nerve cells.
Individual evidence
- ↑ a b c d e data sheet U0126, 99 +% from AlfaAesar, accessed on January 13, 2015 ( PDF )(JavaScript required) .
- ↑ a b Data sheet U0126 monoethanolate, ≥98% (HPLC), powder from Sigma-Aldrich , accessed on January 13, 2015 ( PDF ).
- ↑ MF Favata, KY Horiuchi, EJ Manos, AJ Daulerio, DA Stradley, WS Feeser, DE Van Dyk, WJ Pitts, RA Earl, F. Hobbs, RA Copeland, RL Magolda, PA Scherle, JM Trzaskos: Identification of a novel inhibitor of mitogen-activated protein kinase kinase. In: The Journal of Biological Chemistry . Volume 273, Number 29, July 1998, ISSN 0021-9258 , pp. 18623-18632, PMID 9660836 .
- ↑ JV Duncia, JB Santella, CA Higley, WJ Pitts, J. Wityak, WE Frietze, FW Rankin, JH Sun, RA Earl, AC Tabaka, CA Teleha, KF Blom, MF Favata, EJ Manos, AJ Daulerio, DA Stradley, K. Horiuchi, RA Copeland, PA Scherle, JM Trzaskos, RL Magolda, GL Trainor, RR Wexler, FW Hobbs, RE Olson: MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. In: Bioorganic & Medicinal Chemistry Letters. Volume 8, Number 20, October 1998, ISSN 0960-894X , pp. 2839-2844, PMID 9873633 .
- ↑ H. Fukazawa, K. Noguchi, Y. Murakami, Y. Uehara: Mitogen-activated protein / extracellular signal-regulated kinase kinase (MEK) inhibitors restore anoikis sensitivity in human breast cancer cell lines with a constitutively activated extracellular-regulated kinase ( ERK) pathway. In: Molecular Cancer Therapeutics . Volume 1, Number 5, March 2002, ISSN 1535-7163 , pp. 303-309, PMID 12489846 .
- ↑ P. Bachleda, Z. Dvorák: Pharmacological inhibitors of JNK and ERK kinases SP600125 and U0126 are not appropriate tools for studies of drug metabolism because they activate aryl hydrocarbon receptor. In: General Physiology and Biophysics. Volume 27, Number 2, June 2008, ISSN 0231-5882 , pp. 143-145, PMID 18645229 .
- ↑ T. Smutny, M. Bitman, M. Urban, M. Dubecka, R. Vrzal, Z. Dvorak, P. Pavek: U0126, a mitogen-activated protein kinase kinase 1 and 2 (MEK1 and 2) inhibitor, selectively up -regulates main isoforms of CYP3A subfamily via a pregnane X receptor (PXR) in HepG2 cells. In: Archives of Toxicology . Volume 88, Number 12, December 2014, ISSN 1432-0738 , pp. 2243-2259, doi : 10.1007 / s00204-014-1254-2 . PMID 24819614 .
- ↑ V. Doyère, J. Debiec, MH Monfils, GE sheep, JE LeDoux: Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. In: Nature Neuroscience . Volume 10, Number 4, April 2007, ISSN 1097-6256 , pp. 414-416, doi : 10.1038 / nn1871 . PMID 17351634 .
- ^ Q. Ong, S. Guo, K. Zhang, B. Cui: U0126 Protects Cells against Oxidative Stress Independent of Its Function as a MEK Inhibitor. In: ACS Chemical Neuroscience . [electronic publication before going to press] January 2015, ISSN 1948-7193 , doi : 10.1021 / cn500288n . PMID 25544156 .
- ↑ C. Evans, SJ Cook, MP Coleman, J. Gilley: MEK inhibitor U0126 reverses protection of axons from Wallerian degeneration independently of MEK-ERK signaling. In: PLOS ONE . Volume 8, number 10, 2013, ISSN 1932-6203 , p. E76505, doi : 10.1371 / journal.pone.0076505 . PMID 24124570 . PMC 3790678 (free full text).
