Universal indicator
In chemistry , a universal indicator is a pH indicator that shows pH values over a very wide range through a wide range of color changes.
In most cases, it is not a single substance, but a mixture of several indicator substances, each with a different color and different transition areas, which is coordinated so that the product changes its color with every pH unit. One possibility is a mixture of different amounts of thymol blue , bromothymol blue , methyl red and phenolphthalein .
The universal indicator is therefore not intended for titrations where a transition point that is as precisely defined as possible is desired - individual substances such as methyl orange or phenolphthalein are used here . Rather, they are suitable for roughly determining an unknown pH value in the range from 0 to 14.
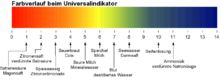
So-called indicator papers are obtained by impregnating filter papers with indicator solutions. In the case of universal indicators, one speaks of universal indicator paper. This is often commercially available as strips in rolls but also in other cuts. With moistened universal indicator paper, gases can also be detected, such as ammonia in the cross match . The following table shows an example of a typical universal indicator, the colors that occur depending on the pH value:
PH value description colour <3 strongly acidic solution red 3-6 acidic solution Orange / yellow 7th neutral solution green 8-11 basic solution (lye) blue > 11 strongly basic solution (strong lye) violet
A mixture of equal parts of methyl red , methyl yellow , thymol blue and bromothymol blue changes color like a traffic light from red to yellow to green.
A natural universal indicator is contained in red cabbage and thus also in water used to cook red cabbage. It is the dye cyanidin (in red cabbage combined with sugar residues as anthocyanidins ), which is red in acid (therefore red cabbage when prepared with vinegar), in weakly basic blue ( blue cabbage made with soda ) and green and yellow in strongly basic in color. In practice, however, this indicator is only suitable for roughly estimating the prevailing milieu.
Individual evidence
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, pp. 574-575.
- ↑ Merck Millipore: pH indicator paper pH 1–10 universal indicator