Weiss reaction
The Weiss reaction is a name reaction from the field of organic chemistry for the synthesis of bicyclic diketones. The reaction is named after its discoverer Ulrich Weiss (1908–1989). Since the Weiss reaction is a condensation reaction and Weiss and his student James M. Cook (* 1945) worked on the synthesis of bicyclic diketones, the terms Weiss-Cook condensation , Weiss-Cook , are used in the literature Reaction and Weiss-Cook process . The reaction is characterized by the formation of four carbon-carbon bonds and the use of simple starting materials that react to form complex products.
Overview reaction
The Weiss reaction is a chain of two aldol reactions followed by two Michael reactions . A cis- bicyclo [3.3.0] octane-3,7-dione is formed from a 1,2-dicarbonyl compound and two equivalents of 3-oxoglutaric acid diesters (e.g. 3-oxoglutaric acid dimethyl ester) in a basic or weakly acidic buffer solution .
In the overview reaction, the 1,2-dicarbonyl compound is symmetrical. If instead, as in the example below, an unsymmetrical 1,2-dicarbonyl compound is used as the starting material, the product is a bicycle with two different radicals R (H, organyl group such as alkyl group ).
Reaction mechanism
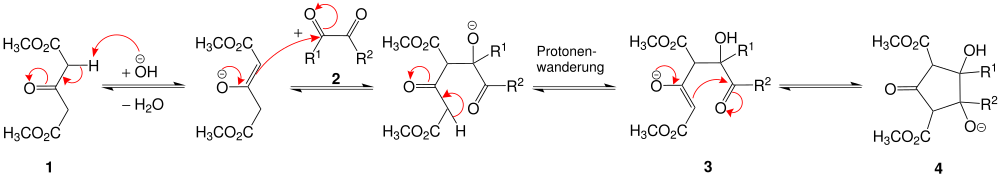
The mechanism has not yet been fully proven, but can be discussed as follows: First there is an intermolecular aldol addition of the 3-oxoglutaric acid dimethyl ester 1 to the 1,2 dicarbonyl compound 2 , in which case R 1 = R 2 = H. An aldol 3 is formed . The subsequent intra- molecular aldol addition results in a ring-shaped intermediate 4 .
After protonation and twice dehydration , the second equivalent of the 3-oxoglutaric acid dimethyl ester can be linked to intermediate 5 by an intermolecular Michael reaction (1st Michael addition) . Immediately followed by a intra -molecular Michael reaction (2. Michael addition) through which the cis -bicyclo [3.3.0] octane-3,7-dione 6 is formed.
The four ester groups of the product can be removed by acid hydrolysis and subsequent decarboxylation and thus replaced by hydrogen atoms.
Stereochemistry
In this reaction only cis isomers are formed , since they are 25.5 kJ / mol more stable than the trans isomers in the sequence of thermodynamic equilibria .
example
In one example, two equivalents of the 3-oxoglutaric acid dimethyl ester react with 1,2-cyclopentanedione - a cyclic 1,2-dicarbonyl compound - to form a complex ring system:
Instead of 1,2-cyclopentanedione, many other acyclic , cyclic or bicyclic 1,2-dicarbonyl compounds can also be used as starting material in the Weiss reaction.
Individual evidence
- ↑ Louis Harris, Ph.D .: Problems of Drug Dependence 1990, Proceeding of the 52nd Annual Scientific Meeting, The Committee on Problems of Drug Dependence, Inc. In: National Institute on Drug Abuse (Ed.): National Institute on Drug Abuse Researches 105 . Alcohol, Drug Abuse and Mental Health Administration, 1991, pp. 130 (DHHS Publication No. (ADM) 91-1753).
- ↑ University of Wisconsin-Milwaukee ( Memento of the original from December 16, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, 2009, ISBN 978-0-471-70450-8 , pp. 2968-2973 .
- ↑ a b T. Laue, A. Plagens: Name and catchword reactions of organic chemistry . 5th edition. BG Teubner, Wiesbaden 2006, ISBN 978-3-8351-0091-6 , p. 339-341 .
- ↑ S. Yang-Lan, M. Mueller-Johnson, J. Oehldrich, D. Wichmann, JM Cook, U. Weiss: Reactions of Dicarbonyl Compounds with Dimethyl β -Ketoglutarate. 4. Formation of 1: 1 adducts . In: J. Org. Chem. Band 41 , no. 26 , 1976, p. 4053-4054 , doi : 10.1021 / jo00888a001 .
- ↑ Bradford P. Mundy, Michael G. Ellert, Frank G. Favaloro, Jr .: Name Reactions in Organic Synthesis , Wiley & Sons, 2nd Edition, 2005, ISBN 0-471-22854-0 , pp. 682-683.
- ↑ R. Mitschka, J. Oehldrich, K. Takahashi, JM Cook, U. Weiss, JV Silverton: General Approach for the Synthesis of Polyquinanes. Facile Generation of molecular Complexity via Reaction of 1,2-Dicarbonyl Compounds with Dimethyl 3-Ketoglutarate . In: Tetrahedron Letters . tape 37 , no. 25 , 1981, pp. 4521–4524 , doi : 10.1016 / j.bbr.2011.03.031 .
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, 2009, ISBN 978-0-471-70450-8 , pp. 2971 .
- ^ Jie Jack Li: Name Reactions - A Collestion of Detailed Mechanisms and Synthetic Applications . 4th edition. Springer, Berlin Heidelberg 2009, ISBN 978-3-642-01052-1 , p. 568-569 .
- ^ T. Laue, A. Plagens: Name and keyword reactions of organic chemistry . 5th edition. BG Teubner, Wiesbaden 2006, ISBN 978-3-8351-0091-6 , p. 339-340 .
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, 2009, ISBN 978-0-471-70450-8 , pp. 2968-2971 .
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, 2009, p. 2968-2969 .
- ↑ S. Yang-Lan, M. Mueller-Johnson, J. Oehldrich, D. Wichmann, JM Cook, U. Weiss: Reactions of Dicarbonyl Compounds with Dimethyl β -Ketoglutarate. 4. Formation of 1: 1 adducts . In: J. Org. Chem. Band 41 , no. 26 , 1976, p. 4053 , doi : 10.1021 / jo00888a001 .
literature
- Jie Jack Li: Name Reactions for Carbocyclic Ring Formations . Wiley New Jersey 2010. ISBN 978-0-470-08506-6