multiple sclerosis
| Classification according to ICD-10 | |
|---|---|
| G35 | Multiple sclerosis ( encephalomyelitis disseminata ) |
| ICD-10 online (WHO version 2019) | |
The multiple sclerosis (MS) or disseminated encephalomyelitis (ED) is an autoimmune , chronic inflammatory neurological disease with different progressive forms, which is why it has been dubbed "the disease with a thousand faces". It attacks the medullary sheaths , the electrically insulating outer layer of the nerve fibers in the central nervous system (CNS).
The exact causes of this demyelinating disease have not yet been clarified, despite great research efforts. In multiple sclerosis, many (multiple) inflammatory foci of demyelinating occur in the white matter of the brain and spinal cord , which are probably caused by the attack of the body's own immune cells on the myelin sheaths of the nerve cell processes. Because the foci of demyelinating can occur throughout the CNS, multiple sclerosis can cause almost any neurological symptom . Visual disturbances with reduced visual acuity up to blindness and disturbances in eye movements ( internuclear ophthalmoplegia ) are typical, but not specific, for multiple sclerosis. The severity of the patient's disability is often indicated using a scale ( EDSS ).
Along with epilepsy, MS is one of the most common neurological diseases in young adults and of considerable social and medical importance. The disease cannot be cured, but the course can often be favorably influenced by various measures. Contrary to popular belief, multiple sclerosis does not necessarily lead to severe disabilities. Even many years after the onset of the disease, the majority of patients can still walk.
Medical history of multiple sclerosis
There are descriptions from the 13th and 14th centuries that indicate MS, for example in Lidwina von Schiedam . For the first time a more precise description of the disease is in the diary of Augustus Frederick d'Este (1794–1848), a grandson of George III. The records of his illness ranged from 1822 to 1846.
D'Este first describes a temporarily reduced visual acuity for the first time at the age of 28:
“In December 1822, I traveled from Ramsgate to the Scottish Highlands to spend a few days with a relative whom I had feelings for as a son. He passed away when I arrived ... Shortly after the funeral, I was forced to read the letters I had received and to have my reply letters written because my eyes were so damaged that it became indistinct when I fixed small things. Unless I tried to read or write, however, I was in no way aware that my eyesight was impaired. I traveled to Ireland shortly after and my eyes recovered without any treatment and regained their strength and clear vision. "
As a result, other typical symptoms of the disease such as double vision, weakness of the legs and feelings of numbness appeared in relapsing forms:
"17th October 1827. To my surprise I noticed (in Venice) a numbness or indistinctness of sensation in the region of the temple above my left eye. In Florence I began to suffer from a vision disorder: around November 6th the disease increased so much that I saw all things twice. Each eye had its own image. Dr. Kissock believed that excessive bile was the cause: leeches were applied to the temple twice , enemas were given, vomiting was induced, and I was veined twice, which was difficult. The illness of my eyes subsided, I saw all things naturally again in their individual state. I was able to go out and walk. Now a new illness began to show itself: With each passing day I found that my strength was gradually running out. Numbness and sensory disturbances occurred in the tailbone and perineum. Finally, by December 4th, the strength of my legs was almost completely gone. I remained in this extraordinary state of weakness for about 21 days ... "
One of the first medical descriptions of multiple sclerosis is attributed to William MacKenzie (1791–1886). In 1840, the Scottish ophthalmologist reported the medical history of a 23-year-old man who, after initially having impaired vision, had been admitted to London's St Bartholomew's Hospital because of increasing paralysis . In addition, a speech disorder ( dysarthria ) and urinary incontinence developed . However, all symptoms had largely disappeared after two months.
The German doctor Friedrich Theodor von Frerichs first diagnosed the disease in a living patient in 1849.
In 1868 Jean-Martin Charcot described the disease not only clinically, but also pathologically in detail: for example, the distribution pattern of multiple sclerosing foci in the vicinity of the cerebral ventricles and in the brain stem, and microscopically the loss of the myelin sheaths in the area of these foci. He called the disease la sclérose en plaques disséminées .
As early as 1877, the neurologist Julius Althaus (1833–1900) suggested naming the disease after Charcot; However, the deonym Charcot's disease has become uncommon for multiple sclerosis outside of France; it is now mainly used for amyotrophic lateral sclerosis .
Treatment with corticosteroids began in 1960, ACTH in 1961 , beta-interferon in 1995 , and glatiramer acetate in 2001 .
Despite the extensive evidence back then, individual researchers still argued that multiple sclerosis had a psychological cause in the 1960s: it was due to the varied symptoms, a typical personality structure long before the first symptoms appeared and a high proportion of women in the disease cases about a conversion hysteria .
Epidemiology
Disease incidence
Multiple sclerosis is the most common chronic inflammatory disease of the central nervous system in Central Europe . Women are affected about twice as often as men ( gynecotropia ). As recently as 2000, the prevalence of the disease in Germany was 149 people per 100,000 inhabitants, resulting in a total of around 122,000 people. However, the evaluation of the data available to the statutory health insurance funds by the Federal Insurance Office (2014) shows a number of at least 200,000–220,000 people diagnosed with MS.
Comparable figures are given for Austria, resulting in a total of around 8150 patients. No epidemiological studies are available for the whole of Switzerland , but a comparable prevalence was determined for the canton of Bern with 110 patients per 100,000 inhabitants. Around 2.5 million people worldwide have MS.
The disease typically occurs in young adulthood, but 3–5% will develop it in childhood or as adolescents. The episodic course with remissions is typical for multiple sclerosis in children. Recurrences are more than twice as common in children as in adults in the first six years of the disease. The recurrences are more severe than in adults, but the remission is better.
mortality
Mortality (mortality) is not significantly increased, especially in patients who do not have severe disabilities . The life expectancy of MS patients in areas for which a full MS registry exists (such as Denmark and parts of Canada) is below that of non-sick people of comparable age. However, in the last few decades Denmark has seen a significant decrease in mortality.
An exception here is the malignant form of MS, the so-called Marburg variant (according to Otto Marburg ), which mainly affects young patients: This acute, severe form of the disease is very rare. This type of MS has severe relapses right from the start. The rapid course leads within weeks or a few months to a severe disability and often also to the death of the person concerned, the latter especially in cases in which foci occur in the brain stem. It is difficult to differentiate the special malignant form of MS from acute disseminated encephalomyelitis ( ADEM ), but in contrast to ADEM, the Marburg variant often lacks a previous virus infection.
Geographical distribution

| | |
| Source: WHO 2004 |
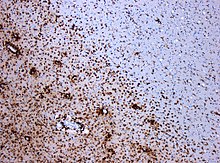
In the immunohistochemical staining for CD68 , numerous macrophages are marked (stained brown) in the area of the lesion.
(Original magnification 1: 100)
In the equatorial zone, the incidence rate is lower than in the northern and southern latitudes. People who move as children or adolescents from MS-rich zones to MS-poor zones (for example from Europe to South Africa or from America or Europe to Israel ) assume the risk of disease in the destination country, while older people keep the disease frequency of their country of origin. This finding represents an important argument for the involvement of an environmental factor in children and adolescents in the later development of the disease ( infection hypothesis ).
Neuropathology
Neuropathologically , MS is characterized by focal (focal), inflammatory-demarking lesions in the CNS with varying degrees of loss of axons and reactive gliosis . Different immunological mechanisms may lead to the loss of the myelin sheath: Histologically, Lassmann et al. Have defined four different subtypes, distinguishing between patients with primarily immunologically induced demyelinating (subtypes I and II) and those with a primary disease of the oligodendroglial cells (subtypes III and IV). It remains unclear whether the characteristics of the subtypes will change in the course of the chronification of the disease. However, evidence of significant differences in the CSF profile between subtype I on the one hand and subtypes II and III on the other - and v. a. the absence or only temporary presence of liquor-specific oligoclonal bands in the last two groups - for the fact that they are actually different immunopathogenic entities. The detection of autoantibodies against human full-length myelin oligodendrocyte glycoprotein in some patients with subtype II lesions, but not in patients with other subtypes, supports this hypothesis, but also suggests heterogeneity within the histological subgroup II. New imaging methods, such as diffusion tensor imaging , but also neuropathological examinations have for some years now increasingly brought the damage to axons in MS back to the fore. The mechanisms that lead to this type of damage are not yet fully understood. In the meantime, it has been concluded that axons can be damaged even if the myelin sheath is still intact. This damage is still reversible in the early stages.
Clinical observations have shown that the individual, inflammatory- demyelinating lesions that cause the relapse-related neurological disorders are not directly related to the chronic progressive disability. To a much greater degree apparently leading atrophy of gray matter to increasing disability. It is known from autopsies that the so-called normal-appearing white or gray matter shows diffuse pathological changes. In patients with extensive demyelination, the gray matter shows a neuronal density reduced by about 20% compared to healthy cortical tissue . The loss of volume in the nervous tissue can occur before other MS symptoms and can progress even as the disease improves clinically. With the new magnetic resonance techniques such as magnetization transfer imaging (MTR) any last doubts have been dispelled that the neural destruction is not limited to individual lesions of the white matter but occurs diffusely throughout the CNS, including the gray matter. The disorders that cause the progressive disability in multiple sclerosis are not only the result of an oligodendrocyte deficiency, they are much more complex.
Causes and origins
The etiology (cause) of MS is unknown. There are numerous theories regarding pathogenesis (origin). The available findings indicate a multifactorial development of the disease with the involvement of genetic factors and environmental influences as the trigger of immune-mediated damage.
Genetic predisposition
MS is not a hereditary disease in the classical sense. By October 2013, 110 genetic variations had been identified that occur more frequently in patients than in the general population and which may contribute to a predisposition to multiple sclerosis. Although each of these variants alone represents a very small risk of developing multiple sclerosis, together they make up about 20 percent of the genetic components of the disease. Among other things, polymorphisms of genes involved in the interleukin signaling pathway are of scientific interest. Many of the gene variants found are directly related to the immune system (e.g. the TNFR 1 variant rs1800693 , the HLA type HLA-DRB1 * 15: 01 ) some of them could also be genetic in autoimmune diseases such as type 1 diabetes or Crohn's disease Risk factors are identified. For the ATP-sensitive potassium channel KIR4.1 on the cell membrane of glial cells, IgG autoantibodies could also be detected in some patients (46.9%).
The risk of disease also depends on ethnicity . Epidemiological studies from the United States indicate that multiple sclerosis is less common there among Hispanics and African Americans .
The risk of developing multiple sclerosis is around 35% in identical twins of MS patients, while the likelihood of developing multiple sclerosis is in siblings (around 4%) and first-degree (around 3%), second-degree (around 1%) or relatives third degree (about 0.9%) is significantly lower. The risk in the general population is approximately 0.1%.
An examination of three identical pairs of twins, of which only one twin had multiple sclerosis, did not reveal any differences on the genetic or epigenetic level; there were also no differences in the transcriptome .
Infection hypothesis
An infection in childhood with a pathogen that shows cross-reactivity with protein components of myelin was suspected as the triggering factor . However, convincing evidence of a specific pathogen has not yet been provided. Studies in adopted children and stepchildren of MS patients speak against the possibility of direct transmission of MS, in which no increased probability of the disease could be demonstrated.
Numerous viruses (including Epstein-Barr virus and human herpes virus 6 ) have been ascribed a possible role in the development of the disease. In fact, especially in children with multiple sclerosis, an immune reaction against the Epstein-Barr virus can be detected more frequently than in children who are not infected. Bacterial pathogens (including chlamydia , spirochetes , rickettsiae and Streptococcus mutans ) have also been linked to the development of multiple sclerosis.
The increase in the number of cases of illness on the Faroe Islands , which began with the stationing of British troops in 1943 and occurred in four waves, is cited as evidence of a possible infectious cause.
Hygiene hypothesis
It is assumed that there is a connection between the immune system's confrontation with infectious diseases and the resulting reduced susceptibility to multiple sclerosis. Living together with siblings in the first six years of life significantly reduces the risk of developing MS, which is explained by the increased mutual infection of siblings with infectious diseases.
Vitamin D metabolism hypothesis
The less common cases of illness in the equatorial zone are also attempted to be explained by the vitamin D metabolism : In humans, vitamin D is mainly produced by solar radiation in the skin. Being exposed to the sun during childhood and having sufficient vitamin D levels in the blood are said to reduce the risk of developing MS later. The low incidence of MS among traditionally living Greenlandic Inuit was explained by their vitamin D-rich diet. Two of the known gene variants are involved in the vitamin D metabolism. Several studies suggest that supplementing with vitamin D could reduce the risk of developing multiple sclerosis. A causal connection could not be established so far, it could also be a coincidental association.
Environmental toxins
There is little evidence for the often alleged causal relationship between the disease and environmental toxins . A meta-analysis also showed no clear connection between amalgam fillings and the likelihood of falling ill.
Smoke
Whether cigarette smoking increases the risk of MS has been researched for years. It is now becoming clear that smoking before the onset of the disease increases the risk. A meta-analysis showed a 1.2 to 1.5-fold increase in the risk of disease. In a Norwegian study, the risk increased by a factor of 1.81.
Smoking also appears to have a negative effect on the development of a clinically isolated syndrome (CIS) for safe MS. In 129 CIS patients who were observed for 36 months, 75% of the smokers, but only 51% of the non-smokers, were diagnosed with MS. It was also investigated how the consumption of cigarettes affects the progression of the disability in the medium term, and whether it could be related to the forms of the course. The greatest differences were found between patients who never smoked and those who started it very early. Early smokers tend to develop chronic forms more frequently and after a shorter duration of the disease, and the risk of the disability progressing is significantly higher.
It is not yet known which pathological changes caused by smoking can influence the development and progression of MS.
Obesity
Obesity in childhood in particular seems to be another factor in the development of multiple sclerosis in adulthood.
Flammer Syndrome
In a study with 58 multiple sclerosis patients and 259 healthy control persons, six of fifteen symptoms of Flammer's syndrome were reported statistically significantly more frequently by MS patients, namely disturbed heat regulation, cold hands and / or feet, dizziness, reduced thirst, tendency to Perfectionism, low body mass index.
Vaccinations
A causal connection between vaccinations - and in particular the hepatitis B vaccination - and the occurrence of MS cannot be proven. Numerous studies with large patient collectives could not confirm a suspected connection based on individual case reports and studies with small patient collectives. On the contrary, an evaluation of data from insured persons from Bavaria showed that people suffering from MS were vaccinated less often five years before this diagnosis than a comparison group without MS.
Chronic cerebrospinal venous insufficiency
In the 1930s, a hypothesis first emerged that MS was caused by a chronic blood flow disorder in the cervical and thoracic veins. This hypothesis of chronic cerebrospinal venous insufficiency (CCSVI) as the cause of MS could not be confirmed. In the 1980s it was discussed again for a short time, again without any clear results. Since 2008, the hypothesis has again received a lot of attention on the occasion of new studies that report a connection between MS and the occurrence of venous insufficiency detected by Doppler sonography , which would lead to the typical foci of inflammation via a subsequent increased intracranial pressure. The reactions from specialist circles to this remained cautious at first, especially since the results could not be reproduced by other research groups and further studies again raised considerable doubts about the quality of the studies on which this theory was based. The German Society for Neurology (DGN) therefore warned in 2010 "... against senseless and dangerous vascular interventions in multiple sclerosis patients [...]"
These assessments were underpinned in 2013 by a study published in the Lancet , which, in addition to the previously used ultrasound examinations of the veins, used catheter venography , which is regarded as the gold standard for imaging diagnostics of venous stenoses . In this study, a CCSVI was consistently found in two to three percent of the test subjects examined - with no differences between MS patients, their siblings and a healthy control group. According to a review published in 2013, the studies carried out in Germany also did not reveal any evidence of a venous cause for MS, which is why it is still not recommended to use vein dilatations as a therapeutic attempt for MS outside of controlled clinical studies.
Experimental animal models of MS
Experimental animal models are used in MS research to study the mechanisms underlying disease development. The influence of individual factors (such as genes and proteins that play an important role in the immune system) on the development of the disease can be studied by means of targeted variation of the experiments. New active ingredients with a therapeutic approach are also initially tested in animal models. The most important animal model for MS is experimental autoimmune encephalomyelitis (EAE). The disease is being studied primarily in specific strains of mice and rats. In addition to many pathological and immunopathogenetic similarities, EAE also shows important differences to MS, so that it must not be equated with MS. It was found that EAE does not depict the complex pathology of MS. However, different variants of EAE reflect individual immunopathogenetic aspects of MS and can be used to investigate specific questions.
Influence of the microbiome
Various studies have shown that the microbiome has an impact on the immune system and on autoimmune diseases. There are intestinal bacteria that dampen the immune system and some that activate it. One publication states that the breakdown products of certain intestinal bacteria are important for the nervous system and need further research.
A study claims to have found an unfavorable composition of the microbiome in people with MS. She suspected that this is the cause of the disease.
The use of antibiotics is considered a risk factor by some researchers.
Microbiome and short chain fatty acids
In previously untreated MS patients, a significant reduction in short-chain fatty acids ( e.g. propionic acid ) was found in the blood and stool (especially after the first attack) . In a new study, these patients were given oral propionate in addition to immunotherapy . This revealed a favorable change in the cell types of the intestinal immune system, which was related to a genetic change in the microbiome. This obviously led to a positive influence on the entire immune system of the patient, who showed a milder course of the disease after further treatment with additional propionate (reduction in the number of attacks, improvement of the overall clinical picture).
Gradient forms
Multiple sclerosis has different forms. The term episode is important for understanding the disease and the forms it takes . A flare-up is defined as the occurrence of new clinical symptoms or the flare-up of known clinical symptoms that persist for more than 24 hours and are based on inflammatory-demineralizing damage to the CNS. New symptoms in MS typically appear subacute , i.e. within hours to days. In order to be able to differentiate a new episode from a previous one, by definition there must be at least 30 days between the two clinical events. The duration of an attack is usually a few days to a few weeks. Depending on whether the symptoms regress completely or only incompletely, one speaks of a complete or incomplete remission. So-called pseudo-relapses are to be distinguished from real relapses, which occur as part of an increase in temperature ( Uhthoff phenomenon ) or are associated with infections and which can lead to a temporary worsening of clinical symptoms.
A distinction is made between the following forms:
Relapsing-remitting MS (RRMS) and secondary progressive MS (SPMS)
In relapsing remitting MS, RRMS or RR-MS for short, individual relapses can be identified that regress completely or incompletely. The RRMS can in the course of disease (approximately 50% of cases after 10 to 15 years) in a secondary progressive MS (English secondary progressive MS, short SPMS or SPMS) merge, the neurological dysfunction by a slow increase is characterized. In addition, relapses can also have a negative impact on the slowly progressing course.
Some factors have been shown to increase the likelihood of individual relapses - these are known as trigger factors . It is considered certain that in the immediate period after an infection (such as the flu or infections of the gastrointestinal tract caused by viruses ) the likelihood of relapse is increased.
During pregnancy , the risk of relapse (especially in the third trimester ) is significantly reduced compared to the disease activity in the previous year. In the three months following the delivery, however, it is increased. In the course of the following 21 months, the disease activity does not differ from the situation before pregnancy.
The influence of psychological stress (such as relationship and marital problems, stress at work, loss of a close relative) on the risk of relapse is controversial. Many previous studies on this topic are accused of methodological deficiencies. More recent studies suggest that psychological stress has a small to moderate influence on the likelihood of relapse.
Primary progressive MS (PPMS)
In contrast to the other forms of MS, the primary progressive MS (English primary progressive , PPMS or PP-MS) does not start with relapses, but with a creeping progression of the neurological deficits without regression. However, superimposed relapses can rarely occur in the further course.
At the beginning, RRMS is the most common form with around 85%; PPMS is diagnosed in only 15% of patients. PPMS is more common in older patients than in younger ones.
Symptoms
The first symptoms usually appear between the ages of 15 and 40 as part of a relapse. Some patients are also a little younger. While the relapses usually regress completely at the onset of the disease, neurological deficits increasingly remain in the later course of the disease after relapses. At the onset of the disease, impaired vision and sensitivity are often observed. It is not uncommon for the disease to begin with an isolated symptom, for which the term clinically isolated syndrome (CIS) has become established.
Which symptom occurs in a flare-up depends on the localization of the active demyelinating focus in the central nervous system: Inflammation in the area of the optic nerve ( retrobulbar neuritis ) causes visual disturbances that are noticeable as blurred vision or a milky haze and are also associated with eye pain (typical initial symptom) can. Foci of inflammation in the area of sensitive railway systems can cause sensory disorders such as paresthesia , numbness and pain. The hands and legs (feet and lower legs) are often affected. Pain can also be caused by trigeminal neuralgia , muscle spasms and the Lhermitte symptom . The Lhermitte sign is typical for MS and can be an indication of foci in the neck area of the spinal cord. If the motor system is affected, symptoms of paralysis ( paresis ) of the extremities occur, whereby the patient's ability to move may be additionally restricted due to an abnormal, involuntary increase in muscle tone ( spastic increase in tone ). Foci in the brain stem and cerebellum can lead to eye movement disorders (double vision and nystagma ), swallowing disorders ( dysphagia ), dizziness, as well as impaired movement coordination ( ataxia ) and speech disorders ( dysarthria ). The symptom complex of intentional tremor , nystagmus and choppy language that occurs in demyelinating foci in the area of the upper cerebellar stalk is called the Charcot triad . A temporal pale of the optic nerve papillae, the presence of paraspasticity and the absence of the abdominal skin reflexes are called the Marburg triad . If vegetative centers and tracts are affected, disorders of the control of the bladder and bowel function and sexual dysfunction can occur. For very many patients during increased physical and mental fatigue (occurs Fatigue ) on. This fatigue occurs independently of physical and psychological stress and increases over the course of the day. As with the other symptoms, fatigue can increase in the context of the Uhthoff phenomenon (more pronounced emergence of the symptoms due to a rise in temperature). Not to be neglected are cognitive and mental disorders. Especially disorders of emotion are common. Subcortical dementia can also occur in the late stages .
One means of quantifying patient disabilities is to use the Expanded Disability Status Scale (EDSS). This scale is used to classify the current severity of the person's disabilities, with the impairments in seven neurological systems being determined beforehand. If one considers the entire course of the disease, it is fatigue, disorders of the bladder function as well as disorders of the motor system such as paralysis and spastic increases in tone that often affect the life of those affected most.
Diagnosis
Prior to the imaging era, diagnosis of multiple sclerosis was based primarily on clinical assessment of symptoms and medical history. Today the diagnosis of multiple sclerosis is based on standardized diagnostic criteria . The most recently revised version of the McDonald criteria serves as the basis for the diagnosis .
Clinical diagnostic criteria
The main clinical criterion for diagnosing MS remains the detection of spatial and temporal dispersion ( dissemination ) of foci of inflammation. Spatial dissemination means the presence of foci of inflammation in more than one location in the central nervous system. Temporal dissemination means that new foci are added as the disease progresses, which can lead to clinical symptoms. If there are no symptoms in the anamnesis or in the neurological examination for foci detectable in the imaging, the lesions are referred to as clinically silent . Spatial and temporal dissemination of disease centers is typical of MS, but it can also be caused by other diseases. The diagnostic criteria therefore expressly emphasize that the diagnosis of MS must not be made if the symptoms and pathological findings can be better explained by another disease. In addition to the medical history and clinical-neurological examination, a number of additional examinations are carried out to diagnose MS:
Imaging examinations
In the slice images of the brain and spinal cord obtained by means of magnetic resonance imaging (MRT), inflamed and scarred tissue areas can be shown. With the help of a gadolinium-containing contrast agent, such as gadopentetate dimeglumine or gadoteric acid , acute foci of disease can be detected, since in their area, unlike intact tissue, the blood-brain barrier is permeable to contrast agent. Periventricular (around the lateral ventricles ) in the medullary bed of the brain and so-called bar foci are typical of MS.
The MRI scan can make a significant contribution to the diagnosis. According to the McDonald criteria, a diagnosis can also be made without MRI imaging (with two relapses and objectifiable functional failures in at least two neurological systems), however, in many patients with a clinical first event, an MRI is necessary for early diagnosis. With the MRT examination, both the spatial and the temporal dissemination of the inflammation centers in the brain and spinal cord can be detected. The McDonald criteria specify exactly how many foci of inflammation must be detectable in which region of the CNS in order to be able to speak of a positive MRI result with regard to the spatial spread. After the last revision of the McDonald criteria, evidence of a temporal dissemination by means of MRI is now also possible with a single MRI, provided that fresh and old lesions can be recognized at the same time that meet certain additional criteria. MS is usually not diagnosed based on imaging findings alone.
Laboratory chemical investigations
Blood tests
There is no known blood biomarker specific for multiple sclerosis . Common inflammatory parameters such as the number of white blood cells , the sedimentation rate or the C-reactive protein are not necessarily increased in MS even during a relapse event. Whether the serum determination of antibodies directed against myelin basic protein (MBP) or myelin oligodendrocyte glycoprotein (MOG) can contribute to the diagnosis remains controversial.
CSF diagnostics
In contrast, in the cerebrospinal fluid , more than 95% of patients have a pathological finding. A lumbar puncture is therefore recommended if the disease is suspected . In 50% of patients there is a slight increase in lymphocytic cells in the CSF (lymphocytic pleocytosis ). An intrathecal antibody synthesis with evidence of oligoclonal bands (OKB) in the isoelectric focusing as an indication of a chronic inflammatory process in the central nervous system is detectable in over 95% of patients. However, the exact sensitivity of the test depends on the testing laboratory. An intrathecal synthesis of antibodies against measles, rubella and varicella-zoster viruses (MRZ reaction) is found in 89% of patients; a so-called 'bi- or trispecific response', d. H. an intrathecal synthesis of antibodies against at least two of the three named pathogens is found in approx. 67% of all adult MS patients. A positive probability ratio of 25.1 was reported for the bi- or trispecific MRZ reaction , i.e. H. A positive result increases the pre-test odds for the diagnosis of MS by the factor mentioned; the specificity is given as 97.5%. The findings listed here (lymphocytic pleocytosis, OKB, MRZ reaction) are typical for MS, but do not in themselves prove the presence of MS.
Neurophysiological examinations
A prolongation of the latency periods when examining evoked potentials (especially the visually and somatosensory evoked potentials) may indicate the conduction of excitation disturbed by demyelination. In advanced disease, axonal damage can also lead to a reduction in amplitude.
Differential diagnosis
The differential diagnosis , i.e. the differentiation of MS from other clinical pictures, covers a large number of diseases. In addition to infectious diseases (especially neurosyphilis , neuroborreliosis or HIV infection ), other chronic inflammatory diseases (such as collagenosis , vasculitis ) must also be excluded. Other inflammatory demyelinating diseases (for example neuromyelitis optica , tropical spastic paraparesis or acute disseminated encephalomyelitis (ADEM)) should also be considered. Metabolic diseases (such as leukodystrophies ) can also lead to symptoms and especially imaging findings similar to those of MS. A deficiency in vitamin B12 can mimic symptoms of MS in the context of funicular myelosis . The possibility that the complaints are based on psychiatric illnesses must also be considered.
The diagnosis of an "unclear stroke" in young patients can be a diagnosis of embarrassment ; MS must also be considered in the differential diagnosis .
Mandatory laboratory tests in the diagnostic phase include C-reactive protein (CRP), complete blood count, blood sugar, vitamin B12, rheumatoid factor , antinuclear antibodies (ANA), antiphospholipid antibodies , lupus anticoagulant, angiotensin-converting enzyme (ACE), Borrelia serology and Urine status . Optionally, if a differential diagnosis is clinically possible, the following are carried out: Anti-neutrophil cytoplasmic antibodies (ANCA), Extractable Nuclear Antigens (ENA), HIV serology, human T-lymphotropic virus 1 (HTLV-1) serology, Treponema pallidum hemagglutination assay (TPHA), long-chain fatty acids, mycoplasma serology.
therapy
Multiple sclerosis is currently not curable. The aim of all therapeutic measures is to maintain the patient's independence in everyday life and to ensure the best possible quality of life. The existing therapeutic options can be divided into relapse therapy , immunomodulatory long-term therapy and the treatment of symptomatic complaints. There is also a focus on preventing complications of MS that can arise, for example, as a result of the patient's immobility. Achieving these therapy goals requires good cooperation between the patient, the caregiver, the patient's environment, neurologist, family doctor, physiotherapist, occupational therapist and representatives from other disciplines. The selection of therapeutic measures always takes into account the individual case of the patient.
Relapse therapy
Relapse therapy is indicated in the event of functional impairment in the patient. In the case of purely sensitive relapses, relapse therapy is usually not necessary. The administration of high-dose therapeutic glucocorticoids can initiate and accelerate the regression of symptoms during an episode. Glucocorticoids have anti-inflammatory effects. Among other things, they reduce the permeability of the blood-brain barrier , so that fewer white blood cells can migrate to the inflammation centers in the CNS. To date, there is no study-based evidence that therapeutic glucocorticoids have a positive effect on the long-term course of the disease.
Intravenous therapy with 1000 mg methylprednisolone over three (to five) days is usual . If, after the first pulse therapy, the effects of an attack are still relevant after at least two weeks, the German Multiple Sclerosis Society recommends that a second pulse therapy with an increased dose of up to five days per 2000 mg should take place. Common side effects of glucocorticoid therapy are sleep disorders and mood swings. Since the glucocorticoids are only administered for a short time, there are no side effects that are typical for long-term therapy with glucocorticoids ( e.g. Cushing's syndrome ).
If the second pulse therapy does not work satisfactorily either, plasmapheresis can be considered to end an acute attack . The use of plasmapheresis is mainly considered for relapses that severely impair the function of the patient (for example, paralysis). In about 40% of patients, plasmapheresis can improve the symptoms. Their implementation is reserved for specialized centers, as possible complications are disorders of the cardiovascular system and infections, which in rare cases can take a serious course.
Course-modifying therapy
Immunomodulation and immunosuppression
The terms immunomodulation and immunosuppression are not always clearly delimited in the literature. Immunomodulating therapies that do not have an immunosuppressive effect are betaine interferons and glatiramer acetate . Immunomodulating therapies that have an immunosuppressive effect are alemtuzumab , cladribine , fingolimod , dimethyl fumarate , natalizumab and teriflunomide (see also leflunomide ).
Classic immunosuppressants such as azathioprine , cyclophosphamide , methotrexate and mitoxantrone can be used for reserve therapy in multiple sclerosis . The aim of long-term therapy with these substances is to prevent new neurological deficits and to delay the worsening of existing deficits. At the pathophysiological level, the active ingredients used are intended to prevent axonal damage by dampening the inflammatory reaction in the CNS. The immunosuppressants do this by stopping the number of white blood cells from multiplying. The principles of action of the immunomodulating substances are diverse and not fully understood. The monoclonal antibody natalizumab was specifically developed as an active ingredient to prevent the migration of white blood cells into the CNS. Medicines that are immunosuppressive can increase the risk of infections (in rare cases serious infections such as PML and opportunistic infections) and cancer. This has not yet been shown in MS therapies that do not have an immunosuppressive effect. In general, the various MS drugs also differ in their safety profile when used during pregnancy.
The basis of treatment in German-speaking countries is the therapy recommendation of the "German Society for Neurology", to which leading researchers and specialized doctors from Germany, Austria and Switzerland belong. The choice of therapy depends initially on whether it is a relapsing or primarily progressive form (PPMS) or secondary progressive form (SPMS) of the disease.
As an addition to immunotherapy, the administration of short-chain fatty acids , which should bring about a more favorable course of the disease , has recently been discussed.
Shear progression (RRMS)
| Active ingredient | Brand name |
Remission rate vs.
Placebo group |
Publications |
|---|---|---|---|
| Basic therapy (according to the guidelines of the MSTKG ) | |||
| Beta interferons | Avonex, Betaferon, Extavia, Rebif | 10% | |
| Dimethyl fumarate | Tecfidera | 9% | |
| Glatiramer acetate | Copaxone | 7% | |
| Alternative therapy (for contraindications to basic therapy) | |||
| Azathioprine | Imurek | ||
| Immunoglobulins | Gamunex 10%, Octagam | ||
| Escalation therapy | |||
| Alemtuzumab | Lemtrada | n / A | |
| Cladribine (tablets) | Mavenclad | n / A | |
| Fingolimod | Gilenya | 7% | |
| Natalizumab | Tysabri | 12% | |
| Ocrelizumab | Ocrevus | n / A | |
| Mitoxantrone | Ralenova | 14% | |
| Cyclophosphamide | Endoxane | ||
In principle, the earliest possible therapy is recommended for RRMS in order to limit axonal damage in the early stages of the disease. This approach is also supported by the fact that the early phase of MS is usually characterized by a particularly high level of inflammatory activity in the CNS.
Basic therapy : Equal therapeutic agents of first choice are beta-interferon preparations , dimethyl fumarate , glatiramer acetate and teriflunomide (also fingolimod in Switzerland ). These therapies are called basic therapies - for mild / moderate forms. If one of these therapies leads to rapid progression of the neurological deficits, you can switch to another basic therapy or escalation therapy (second-line therapy).
Since autumn 2019, beta interferons (e.g. Rebif ) have also been approved for those who are pregnant or wanting to have children . If clinically necessary, therapy need not be interrupted or postponed because of pregnancy. Since no harmful effects are to be expected for the child, breastfeeding is possible without restriction under interferon beta.
Escalation therapy: The first choice agents used in escalation therapy are alemtuzumab , cladribine , fingolimod , natalizumab and ocrelizumab . Second choice active ingredients are mitoxantrone and, in rare cases, cyclophosphamide . Meta-analyzes could not provide convincing proof of efficacy for all preparations .
In August 2017 was cladribine (brand name: Mavenclad , Merck KGaA ) in the EU - as oral pulse therapy with long-acting (tablets) - for the treatment of RMS (RRMS and SPMS) in patients with high disease activity permitted. It was approved in the USA in March 2019.
Side effects : Therapy is generally continued as long as there is a positive effect on the development of MS and no serious side effects occur. As a result, daclizumab (approved in the EU in July 2016) was withdrawn from the market in March 2018 . If there are contraindications to these agents, azathioprine and intravenous immunoglobulins can be used as agents. Due to severe dose-dependent side effects (mitoxantrone is cardiotoxic ), there is a limited lifetime dose for mitoxantrone , which is reached after about two to five years. Treatment with beta interferons and natalizumab can lead to the development of neutralizing antibodies (nAb). While a possible loss of effectiveness due to nAbs for the beta interferons is controversial, nAbs seem to actually reduce the effectiveness of natalizumab.
A serious side effect of fingolimod , for which the immunosuppressive effect is blamed, is progressive multifocal leukoencephalopathy (PML) . In November 2017, the manufacturer of fingolimod ( Novartis ) published numerous cardiac contraindications in a so-called Rote-Hand-Brief (RHB) for which fingolimod ( GILENYA ) may not be prescribed / used. In September 2019, Novartis published another RHB on a new contraindication for use during pregnancy and in women of childbearing potential who are not using effective contraception.
The European Medicines Agency (EMA) has been investigating several cases of serious, sometimes fatal side effects with alemtuzumab since April 2019 . In the meantime, the pharmaceutical company (Sanofi Genzyme) has also issued various Rote-Hand-Letters (RHB) for alemtuzumab, etc. a. published in April 2019 and January 2020.
To cladribine tablets had existed since the approval in September 2017 for the treatment of MS no Red Hand Letter. At the world's largest annual international congress devoted to basic and clinical research in multiple sclerosis, ECTRIMS, it was stated in 2019 that no relevant side effects had been found until then, and no PML cases had occurred either.
The beta interferon preparations and glatiramer acetate are also approved for the treatment of Clinically Isolated Syndrome (CIS) under certain conditions .
Chronically progressive forms
For the treatment of secondary progressive MS (SPMS) , subcutaneously administered beta interferon 1a (if there is relapse activity) and beta interferon 1b can be used. The drug mitoxantrone, which has been approved for this indication since 2002, can also be used. After the maximum dose of mitoxantrone has been reached and disease activity persists, therapy attempts with quarterly high-dose intravenous glucocorticoid jolts (usually methylprednisolone ) or cyclophosphamide can be attempted.
For the treatment of primarily chronic progressive MS (PPMS) , but also relapsing MS, ocrelizumab was approved by the FDA in the USA in March 2017 with the condition that some phase IV studies be carried out, and in September 2017 by Swissmedic also in Switzerland. The EU approval took place in January 2018.
Hit-hard-and-early strategy
Since the effectiveness of the basic therapy (see above) is associated with only approx. 30 - 40% relapse reduction - compared to the (highly) active therapies of escalation therapy (see above) in the amount of approx. 50 - 70% reduction in relapse rate, this continues called hit-hard-and-early strategy more and more: Experts recommend starting with the drugs of escalation therapy right at the beginning.
pipeline
Numerous substances are currently in clinical development ( phase III ), e.g. E.g .: the so-called small molecules ozanimod ( Bristol-Myers Squibb or Celgene ) and siponimod ( Novartis ). Siponimod has now been approved. There is also a Brutontyrosine Kinase Inhibitor (BTKi) Evobrutinib z. B. still in clinical development.
Symptomatic therapy
Many symptoms during the course of MS can lead to a decrease in the quality of life. The respective functional disorders and their extent are different in each patient. Particularly common and limiting are mobility problems , spasticity , pain , bladder dysfunction , speech and swallowing disorders, a quicker mental and physical fatigue ( Fatigue ) and depressive disorders . In addition to lifestyle changes, physiotherapeutic , speech therapy , occupational therapy, psychotherapeutic, medicinal and surgical measures are suitable for treating these symptoms . The prophylaxis of serious complications such as aspiration pneumonia , pulmonary embolism , thrombosis , osteoporosis , decubitus ulcers , joint contractures , urinary tract infections and desiccosis (dehydration) is particularly important . These complications are partly responsible for the reduced life expectancy in MS patients compared to the general population.
Treatment of walking difficulties
If MS is more advanced, people may develop walking difficulties that can be improved by physical therapy and certain, such as B. antispasmodic drugs can be treated. If the walking disability is of a certain severity, treatment with fampridine (trade name Fampyra) is an option . Fampridine has been approved in Germany since 2011 for patients with a higher degree of walking disability as a result of multiple sclerosis (grade 4–7 on the EDSS disability scale). Fampridine is a potassium channel blocker. It acts on damaged nerves, where it prevents charged potassium particles from escaping from the nerve cells. It is believed that this allows the electrical impulses to travel further down the nerves to stimulate the muscles. This makes walking easier.
Treatment of spasticity
Spastic increases in muscle tone are caused by foci in the pyramidal tract . They can directly cause pain or a feeling of tension or lead to pain through secondary diseases such as muscle and joint contractures, deformities and immobility. A physiotherapy is always advised when pathological Tonuserhöhungen. Using the Bobath concept , the tonic increased muscles can be inhibited (inhibited) and detonated muscles and movement coordination can be activated or facilitated. Orally, for example, baclofen or tizanidine can be used as medication . Localized spastic increases in tone can also be treated with injections of botulinum toxin . Another option is the administration of baclofen or triamcinolone directly into the subarachnoid space in the area of the lumbar spine (intrathecal application). A preparation with the active ingredients tetrahydrocannabinol (THC) and cannabidiol has been approved in Germany as an add-on therapy for moderate and severe forms of spasticity since 2011 .
In the context of “ off-label use ” (i.e. outside of the use approved in the approval), gabapentin can be used for spasticity if the substances approved for this cannot provide adequate relief with appropriate dosage and duration of use or if there is intolerance. A resolution of the Federal Joint Committee (G-BA) on the prescribability in an unauthorized area of application came into force in March 2014.
Pain management
Pain in MS patients can have a variety of causes. Trigeminal neuralgia , which is caused directly by foci of inflammation and occurs in attacks, can be treated with medication with carbamazepine , gabapentin or pregabalin . Chronic pain, mostly in the extremities, probably caused by foci in the spinal cord, is caused by MS itself and can be treated with amitriptyline , for example . Pain can also be caused indirectly by consequences of MS, such as increased spasticity of the extremities or urinary tract infections. The therapy in these cases depends on the cause.
Treatment of bladder dysfunction
Bladder dysfunction manifests itself in urinary tract infections, imperative urination, pollakiuria and incontinence. The underlying disorders may be a storage disorder, a voiding disorder or a detrusor-sphincter dyssynergy of the urinary bladder. After specific urological diagnostics, an appropriate therapy with a division of the fluid supply, pelvic floor exercises , catheterization and medication can take place. Urinary tract infections must be treated with antibiotics. Desiccation caused by patients drinking less to minimize bladder disorders must be avoided.
Treatment of speech and swallowing disorders
Speech and swallowing disorders can put the patient under considerable strain. Acute disorders that arise during an episode are treated using episode therapy. If the symptoms persist, speech therapy measures are mainly used. In the case of pronounced swallowing disorders, parenteral nutrition and the creation of a PEG may also be necessary. The goals here are adequate food intake and avoidance of aspiration pneumonia.
Treatment of fatigue and depressive disorders
The diagnostic criteria for fatigue and depression contain similar elements. Many patients have both. A depressive disorder can be treated with antidepressants, for example from the group of so-called selective serotonin reuptake inhibitors (SSRI). Psychological support can also help treat secondary depressive disorders and better cope with the consequences of the illness. In addition to antidepressants, amantadine , acetyl-L-carnitine, acetylsalicylic acid and modafinil can also be used for drug treatment of fatigue syndrome . However, the effectiveness of some of these preparations for this indication is not undisputed.
Treatment of sexual disorders
50 to 90% of MS patients report sexual disorders in the course of the disease, with men appearing to be more frequently affected. Inflammatory foci can be the direct organic cause of the disorders, in that they lead to sensory disorders in the genital area or impair the reflex arcs of the sexual functions (for example for erection ). Spasticity of the thigh muscles of the legs or the muscles of the pelvic floor, which occurs as a result of MS, can also make sexual intercourse difficult or impossible. Reduced lubrication can lead to pain during intercourse. Furthermore, all influences that affect the patient in his social, psychological and existential structure due to his illness can lead to disorders of sexuality. Fatigue or a depressive episode can be accompanied by a loss of libido . Social conflict, isolation, and shame can also affect sexuality. The aim of therapeutic sexual counseling is to inform the patient (and his partner) about possible causes of the disorders and to develop and point out possible solutions in conversation. Organic causes can be treated by optimizing the appropriate symptomatic therapy. Erectile dysfunction can be treated with phosphodiesterase inhibitors such as sildenafil , tadalafil or vardenafil , unless they are mainly of psychological origin . Furthermore, aids such as lubricants with low lubrication and vibrators can be used for sexual stimulation. In addition, many drugs that are used as part of symptomatic therapy can lead to loss of libido and sexual dysfunction.
nutrition
A meta-analysis showed no evidence of a significant effect of increased consumption of polyunsaturated fatty acids (e.g. omega-6 fatty acids such as linoleic acid or omega-3 fatty acids ) on the course of the disease.
Therapies outside of evidence-based medicine
Many MS patients use complementary or alternative medical treatments in addition to or instead of evidence-based medical therapy. The use of unconventional therapies is more common in patients who are more limited by MS. There is a very large number of offers (such as special diets, acupuncture , homeopathy ). No reliable proof of effectiveness has been produced for any of the unconventional therapy offers.
outlook
In addition to the drugs approved in Germany for the treatment of multiple sclerosis (interferon beta-1b, interferon beta-1a sc, interferon beta-1a im, fumaric acid dimethyl ester, glatiramer acetate, mitoxantrone, azathioprine, fingolimod, natalizumab, alemtuzumab and teriflunomide) there are a large number of active substances that are in different phases of the test. In Germany, patients are currently (as of 2019) being recruited for at least 25 ongoing clinical studies.
An important focus of clinical research is the further development of immunomodulatory active ingredients that prevent the progression of the disability more effectively. Other studies aim to increase the ease of use through longer application intervals or oral administration. The effectiveness and safety of more aggressive forms of treatment that aim to eliminate the disturbed immune system in order to then establish a new, tolerant immune system (either through stem cells remaining in the bone marrow or through reinfusion of autologous stem cells) remains to be clarified in the context of randomized clinical studies but will certainly be reserved for a few specialized centers. According to a clinical study carried out at the Ottawa Hospital Research Institute, the autoimmune reaction has been stopped for up to 13 years by reinfusing autologous stem cells.
Attempts to promote remyelination and regeneration through the use of growth factors or a modulation of stem cells are still an experimental approach.
A study from Rome was able to show that the BCG tuberculosis vaccination in the early stages can prevent the development of new lesions and positively influence the course of the disease. However, too little data is still available to recommend BCG vaccination for all MS patients.
As with other neurodegenerative diseases, treatment approaches with stem cells are also being tested for multiple sclerosis . The interest in mesenchymal stem cells , for example , is based on the following properties and functions of these cells: 1.) They have an immunomodulating and immunosuppressive effect. 2.) They support the restoration of the myelin sheath by nourishing the oligodendrocytes. 3.) They are able to protect nerve tissue via biochemical reactions ( neuroprotection ).
forecast
So far it has hardly been possible to make a prognosis about the further course of the disease at the beginning of the disease, which burdens the affected patients. Several epidemiological studies on the prognosis of multiple sclerosis have been published in recent years. The results were mostly positive and showed that the disease is often less severe than generally assumed. Based on the disease progression of 1059 patients, a Munich working group has developed a web-based computer program to determine the individual risk profile based on the disease progression, expanded disability status scale , duration of illness, frequency of relapses and age.
Others
pregnancy and breast feeding period
In principle, pregnancy is possible if the diagnosis is MS. MS does not seem to affect fertility in women or men. Pregnancy is unlikely to promote the progression of MS. On the contrary: the relapse rate usually decreases continuously during pregnancy. After the birth, however, the risk of MS relapses increases again. In the long term, the relapse rate will return to the same level it was before pregnancy.
If a relapse occurs, it is possible to give high-dose cortisone .
MS is not a "classic" hereditary disease - the predisposition to develop MS can, however, be inherited. According to studies, this means: The risk for a child to develop MS themselves, if one parent with MS is 2%. This is higher than in parents who are not affected by MS (0.1-0.2%). If both parents have MS, the risk for the child increases to 20%.
MS and family planning
The information used in the table below comes from the official reports of the European Medicines Agency (EMA), which is responsible for the authorization, evaluation and monitoring of all medicines in Europe.
| For highly active MS | When can I get pregnant? | Therapy and pregnancy possible? | Therapy and breastfeeding possible? |
|---|---|---|---|
|
Alemtuzumab ( Lemtrada ) |
4 months after an infusion | Infusion during pregnancy only after the benefit-risk assessment with the attending physician However, pregnancy is possible 4 months after an infusion |
Breastfeeding is possible 4 months after the last infusion |
|
Cladribine (tablets) ( Mavenclad ) |
6 months after taking the tablet, if possible after the 2nd year of treatment. In the 3rd and 4th year no tablets are planned. |
Taking tablets during pregnancy is not allowed, consult a doctor immediately if pregnant. However, pregnancy is possible 6 months after the last tablet-taking in year 2. |
Breastfeeding is possible 1 week after taking the last tablet |
|
Fingolimod ( Gilenya ) |
2 months after the last dose. Disease activity may return after discontinuation. | Ingestion during pregnancy is not allowed, consult a doctor immediately if pregnant |
Breastfeeding is not possible during treatment |
|
Mitoxantrone ( Ralenova ) |
6 months after the infusion, risk of infertility | Ingestion during pregnancy is not allowed, consult a doctor immediately if pregnant | Breastfeeding is not possible during treatment |
|
Natalizumab ( Tysabri ) |
No information. Disease activity may return after discontinuation | Use only after the benefit-risk assessment with the attending physician | Breastfeeding is not possible during treatment |
|
Ocrelizumab ( Ocrevus ) |
12 months after the last infusion | Use only after the benefit-risk assessment with the attending physician | Breastfeeding is not possible during treatment |
|
Glatiramer acetate ( Copaxone , Clift ) |
No information | Use only after the benefit-risk assessment with the attending physician | Breastfeeding is only possible after weighing the risks and benefits |
|
Dimethyl fumarate ( Tecfidera ) |
No information | Use only after the benefit-risk assessment with the attending physician | Breastfeeding is only possible after weighing the risks and benefits |
| Teriflunomid (Aubagio) | If teriflunomide concentration falls below 0.02 mg / l after discontinuation. | Use during pregnancy is not allowed, consult a doctor immediately if pregnant |
Breastfeeding is not possible during treatment |
|
Beta interferons ( Avonex , Betaferon , Extavia , Rebif ) |
immediately | if clinically necessary, officially approved | Breastfeeding is possible during treatment. |
Stem cell donation, organ transplant, blood donation
MS (like a number of other diseases) is an exclusion criterion for a stem cell donation . People with MS usually cannot donate organs . Although donating blood from an MS patient is unlikely to involve any significant risk for either the donor or the recipient, the DMSG's medical advisory board advises against this as a matter of principle. Many people with MS receive long-term treatment with immunosuppressive or immunomodulating drugs and are therefore not considered blood donors anyway.
World Multiple Sclerosis Day
Since 2009, World Multiple Sclerosis Day ( World MS Day ) has been celebrated on the last Wednesday in May . Many MS associations and self-help groups, in Germany above all the Deutsche Multiple Sklerose Gesellschaft e. V. (DMSG), hold action events on this day to provide information about multiple sclerosis and its effects and to arouse understanding for the concerns of people with MS. On the occasion of the first World MS Day, the International MS Association MSIF together with the non-profit Hertie Foundation made an awareness film called Beautiful Day. The rock band U2 made their song available for this. The film has been translated into numerous languages and has attracted a lot of international attention for the disease.
literature
- Alastair Compston: McAlpine's Multiple Sclerosis. Churchill Livingstone, Oxford 2005, ISBN 0-443-07271-X .
- Ralf Gold, Peter Rieckmann: Pathogenesis and therapy of multiple sclerosis. Uni-Med, Bremen 2004, ISBN 3-89599-785-4 .
- Volker Limmroth, Eckhart Sindern: Multiple Sclerosis. Thieme, Stuttgart 2004, ISBN 3-13-133281-6 .
- Rudolf M. Schmidt, Frank Hoffmann: Multiple sclerosis. Urban & Fischer, Munich 2006, ISBN 3-437-22081-0 .
- Heinz Wiendl , Robert Weißert, Volker Limmroth, Reinhard Hohlfeld: Multiple sclerosis and other demyelinating diseases. In: Thomas Brandt, Johannes Dichgans , Hans-Christoph Diener (eds.): Therapy and course of neurological diseases. 5th edition. Kohlhammer, Stuttgart 2007, ISBN 978-3-17-019074-0 , p. 654 ff.
Web links
- Link catalog on multiple sclerosis at curlie.org (formerly DMOZ )
- Publications of the magazine MS in Focus can be downloaded free of charge
- MS Resource Center ( Memento from April 30, 2013 in the Internet Archive )
Umbrella organizations
- MS International Federation
- German Multiple Sclerosis Society
- Website of the Swiss Multiple Sclerosis Society
- MS Society Vienna
- Disease-related competence network multiple sclerosis
Guidelines and principles
- S2e guideline multiple sclerosis, diagnosis and therapy of the German Society for Neurology (DGN). In: AWMF online (as of 2012)
- Principles for improving the quality of life of people with multiple sclerosis ( Memento from November 20, 2008 in the Internet Archive )
- S2 guideline multiple sclerosis in childhood of the Society for Neuropediatrics (GNP). In: AWMF online (as of 2008)
Individual evidence
- ↑ Multiple sclerosis: the disease with a thousand faces. In: German Society for Neurology . May 2011, accessed January 23, 2020 .
- ↑ a b c Roland Besser, Günter Krämer : Multiple Sclerosis: Answers to the most common questions. Georg Thieme Verlag, 2006, ISBN 3-8304-3333-6 , p. 14 restricted preview in the Google book search
- ↑ Possible portrait in the Victoria and Albert Museum
- ↑ Pearce: Historical descriptions of multiple sclerosis. Eur Neurol. 2005; 54 (1), pp. 49-53)
- ↑ Firth: The case of August D'Este . Cambridge University Press. Cambridge 1948.
- ^ TJ Murray: Multiple sclerosis: the history of a disease. Demos Medical Publishing, 2005 limited preview in Google Book search
- ^ Translated into German according to WI McDonald: Physicians, subsequence and consequence. In: Journal of Neurology, Neurosurgery, and Psychiatry . Volume 67, Number 3, September 1999, pp. 282-289, PMID 10449547 . PMC 1736520 (free full text).
- ^ MacKenzie: A practical treatise on diseases of the eye. Longman, London 1840; quoted from WI McDonald: Physicians, subsequence and consequence. In: Journal of neurology, neurosurgery, and psychiatry . Volume 67, Number 3, September 1999, pp. 282-289, PMID 10449547 . PMC 1736520 (free full text).
- ↑ DM Bourneville, L. Guérard: De la sclérose en plaques disséminées: nouvelle étude sur quelques points de la sclérose en plaques disséminées par Bourneville . Adrien Delahaye, Paris 1869 ( books.google.cl ).
- ↑ C. Aring: Observations on multiple sclerosis and conversion hysteria . In: Brain: A Journal of Neurology . tape 88 , no. 4 , November 1965, p. 663-674 , PMID 5856073 .
- ↑ T. Hein, W. Hopfenmüller: extrapolation of the number of multiple sclerosis patients in Germany. In: The neurologist . Volume 71, Number 4, April 2000, pp. 288-294, PMID 10795096 .
- ↑ G. Petersen et al .: Epidemology of Multiple Sclerosis in Germany. In: The neurologist . Edition 8/2014, 85: 990-998.
- ↑ U. Baumhackl et al .: Prevalence of multiple sclerosis in Austria. Results of a nationwide survey. In: Neuroepidemiology. Volume 21, Number 5, 2002 Sep-Oct, pp. 226-234, doi: 10.1159 / 000065640 . PMID 12207150 .
- ^ S. Beer, J. Kesselring: High prevalence of multiple sclerosis in Switzerland. In: Neuroepidemiology. Volume 13, Numbers 1-2, 1994, pp. 14-18, PMID 8190201 .
- ↑ Trial of Fingolimod versus Interferon Beta-1a in Pediatric Multiple Sclerosis | NEJM. Retrieved January 4, 2019 .
- ↑ T Chitnis et al .: Demographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United States. In: Multiple Sclerosis . tape 15 , no. 5 , 2009, p. 627-631 , doi : 10.1177 / 1352458508101933 , PMID 19299440 .
- ↑ Janko Samardzic, John N. van den Anker, Sasa Peric, Dejan Stevanovic, Ivan Zaletel: Multiple Sclerosis in Pediatrics: Current Concepts and Treatment Options . In: Neurology and Therapy . tape 5 , no. 2 , December 1, 2016, p. 131–143 , doi : 10.1007 / s40120-016-0052-6 , PMID 27640189 , PMC 5130919 (free full text) - ( springer.com [accessed January 4, 2019]).
- ↑ AJ Fay, EM Mowry, J Strober, E Waubant: Relapse severity and recovery in early pediatric multiple sclerosis. In: Multiple Sclerosis . tape 18 , no. 7 , 2012, p. 1008-1012 , doi : 10.1177 / 1352458511431725 .
- ↑ S. Poser, W. Poser, G. Schlaf, W. Firnhaber, K. Lauer, M. Wolter, P. Evers: Prognostic indicators in multiple sclerosis. In: Acta neurologica Scandinavica. Volume 74, Number 5, November 1986, pp. 387-392, PMID 3825497 .
- ^ BJ Hurwitz: Analysis of current multiple sclerosis registries. In: Neurology . Volume 76, Number 1 Suppl 1, January 2011, pp. S7-13, doi: 10.1212 / WNL.0b013e31820502f6 . PMID 21205683 . (Review article).
- ^ H. Brønnum-Hansen, N. Koch-Henriksen, E. Stenager: Trends in survival and cause of death in Danish patients with multiple sclerosis. In: Brain. Volume 127, Pt 4 April 2004, pp. 844-850, doi: 10.1093 / brain / awh104 . PMID 14960501 .
- ^ W. Hu, CF Lucchinetti: The pathological spectrum of CNS inflammatory demyelinating diseases. In: Seminars in Immunopathology . Volume 31, Number 4, November 2009, pp. 439-453, doi: 10.1007 / s00281-009-0178-z . PMID 19779719 . (Review article).
- ↑ Death and DALY estimates for 2004 by cause for WHO Member States (Excel table; 3.1 MB)
- ^ CR Gale, CN Martyn: Migrant studies in multiple sclerosis. In: Progress in neurobiology. Volume 47, Numbers 4-5, Nov-Dec. 1995, pp. 425-448, PMID 8966212 . (Review article).
- ↑ H. Lassmann, W. Brück, C. Lucchinetti: Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. In: Trends in Molecular Medicine . Volume 7, Number 3, March 2001, pp. 115-121, PMID 11286782 . (Review article).
- ^ S. Jarius, FB König, I. Metz, K. Ruprecht, F. Paul, W. Brück, B. Wildemann: Pattern II and pattern III MS are entities distinct from pattern I MS: evidence from cerebrospinal fluid analysis . In: J Neuroinflammation . 14, 29 August 2017, p. 171. doi : 10.1186 / s12974-017-0929-z . PMID 28851393 .
- ↑ S. Jarius, I. Metz, FB König, K. Ruprecht, M. Reindl, F. Paul, W. Brück, B. Wildemann B: Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in 'pattern II multiple sclerosis' and brain biopsy findings in a MOG-IgG-positive case . In: Mult Scler . 22, No. 12, October 2016, pp. 1541–1549. doi : 10.1186 / s12974-017-0929-z . PMID 26869529 .
- ↑ M. Spadaro, LA Gerdes, MC Mayer, B. Ertl-Wagner, S. Laurent, M. Krumbholz, C. Breithaupt, T. Högen, A. Straube, A. Giese, R. Hohlfeld, H. Lassmann, E Meinl, T. Kümpfel .: Histopathology and clinical course of MOG-antibody-associated encephalomyelitis . In: Ann Clin Transl Neurol . 2, January 14, 2015, pp. 295-301. PMID 25815356 .
- ↑ New approach to therapy for multiple sclerosis: The first nerve damage is still reversible ( Memento from December 27, 2011 in the Internet Archive ) from dmsg.de - accessed on April 7, 2011.
- ↑ FB König, B. Wildemann, S. Nessler, D. Zhou, B. Hemmer, I. Metz, HP Hartung, BC Kieseier, W. Brück: Persistence of immunopathological and radiological traits in multiple sclerosis . In: Arch Neurol . 65, No. 11, November 2008, pp. 1527-1532. doi : 10.1001 / archneur.65.11.1527 . PMID 19001173 .
- ↑ PA Brex et al .: A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. In: The New England Journal of Medicine . Volume 346, Number 3, January 2002, pp. 158-164, doi: 10.1056 / NEJMoa011341 . PMID 11796849 .
- ↑ N. Evangelou et al .: Pathological study of spinal cord atrophy in multiple sclerosis suggests limited role of local lesions. In: Brain: a journal of neurology. Volume 128, Pt 1 January 2005, pp. 29-34, doi: 10.1093 / brain / awh323 . PMID 15548559 .
- ↑ A. Kutzelnigg et al .: Cortical demyelination and diffuse white matter injury in multiple sclerosis. In: Brain: a journal of neurology. Volume 128, Pt 11 November 2005, pp. 2705-2712, doi: 10.1093 / brain / awh641 . PMID 16230320 .
- ^ Lydia Bernhardt: Neuronal Degeneration in Spinal Multiple Sclerosis. Dissertation, Medical Faculty Charité - Universitätsmedizin Berlin, 2010 (PDF)
- ↑ a b c A. Ascherio: Environmental factors in multiple sclerosis. In: Expert review of neurotherapeutics. Volume 13, Number 12 Suppl, December 2013, pp. 3–9, doi: 10.1586 / 14737175.2013.865866 . PMID 24289836 . (Review).
- ↑ SG Gregory et al .: Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. In: Nature Genetics . Volume 39, Number 9, September 2007, pp. 1083-1091, doi: 10.1038 / ng2103 . PMID 17660817 .
- ↑ F. Lundmark et al .: Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. In: Nature genetics. Volume 39, Number 9, September 2007, pp. 1108-1113, doi: 10.1038 / ng2106 . PMID 17660816 .
- ↑ MG Marrosu: Susceptibility to multiple sclerosis: the role of interleukin genes. In: Lancet neurology. Volume 6, number 10, October 2007, pp. 846-847, doi: 10.1016 / S1474-4422 (07) 70228-4 . PMID 17884670 .
- ↑ S. Sawcer: The complex genetics of multiple sclerosis: pitfalls and prospects. In: Brain. Volume 131, Pt 12 December 2008, pp. 3118-3131, doi: 10.1093 / brain / awn081 . PMID 18490360 . PMC 2639203 (free full text). (Review article).
- ^ AP Gregory et al .: TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. In: Nature. Volume 488, number 7412, August 2012, pp. 508-511, doi: 10.1038 / nature11307 . PMID 22801493 .
- ↑ NA Patsopoulos et al .: Fine-mapping the genetic association of the major histocompatibility complex in multiple sclerosis: HLA and non-HLA effects. In: PLoS genetics. Volume 9, number 11, November 2013, p. E1003926, doi: 10.1371 / journal.pgen.1003926 . PMID 24278027 . PMC 3836799 (free full text).
- ↑ a b Genetic analysis brings new insights into the development of multiple sclerosis dmsg.de, on August 11, 2011, accessed on March 9, 2018.
- ^ The International Multiple Sclerosis Genetics Consortium & The Wellcome Trust Case Control Consortium: Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. In: Nature . Volume 476, number 7359, August 2011, pp. 214-219, doi: 10.1038 / nature10251 . PMID 21833088 . PMC 3182531 (free full text).
- ↑ R. Srivastava et al .: Potassium channel KIR4.1 as an immune target in multiple sclerosis. In: The New England journal of medicine . Volume 367, Number 2, July 2012, pp. 115-123, doi: 10.1056 / NEJMoa1110740 . PMID 22784115 .
- ^ R. Schneider: Autoantibodies to Potassium Channel KIR4.1 in Multiple Sclerosis. In: Frontiers in neurology. Volume 4, 2013, p. 125, doi: 10.3389 / fneur.2013.00125 . PMID 24032025 . PMC 3759297 (free full text).
- ↑ M. Alter et al .: Multiple sclerosis frequency in Israel's diverse populations. In: Neurology . Volume 66, Number 7, April 2006, pp. 1061-1066, doi: 10.1212 / 01.wnl.0000204194.47925.0d . PMID 16606919 .
- ↑ DM Williamson et al .: Prevalence of multiple sclerosis in 19 Texas counties, 1998-2000. In: Journal of environmental health. Volume 69, Number 10, June 2007, pp. 41-45, PMID 17583295 .
- ^ GC Ebers et al .: Conjugal multiple sclerosis: population-based prevalence and recurrence risks in offspring. Canadian Collaborative Study Group. In: Annals of Neurology . Volume 48, Number 6, December 2000, pp. 927-931, PMID 11117550 .
- ^ NP Robertson et al .: Age-adjusted recurrence risks for relatives of patients with multiple sclerosis. In: Brain: a journal of neurology. Volume 119 (Pt 2), April 1996, pp. 449-455, PMID 8800940 . doi: 10.1093 / brain / 119.2.449 .
- ^ Daniel S. Reich, Claudia F. Lucchinetti, Peter A. Calabresi: Multiple Sclerosis . Ed .: New England Journal of Medicine. No. 378 , 2018, p. 169-180 .
- ↑ SE Baranzini et al .: Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. In: Nature. Volume 464, number 7293, April 2010, pp. 1351-1356, doi: 10.1038 / nature08990 . PMID 20428171 . PMC 2862593 (free full text).
- ↑ A. Katsnelson: Twin study surveys genome for cause of multiple sclerosis. In: Nature. Volume 464, number 7293, April 2010, p. 1259, doi: 10.1038 / 4641259a . PMID 20428135 . (Comment)
- ^ DA Dyment et al .: Multiple sclerosis in stepsiblings: recurrence risk and ascertainment. In: Journal of neurology, neurosurgery, and psychiatry . Volume 77, Number 2, February 2006, pp. 258-259, doi: 10.1136 / jnnp.2005.063008 . PMID 16421134 . PMC 2077589 (free full text).
- ^ B. Banwell et al .: Clinical features and viral serologies in children with multiple sclerosis: a multinational observational study. In: Lancet neurology. Volume 6, Number 9, September 2007, pp. 773-781, doi: 10.1016 / S1474-4422 (07) 70196-5 . PMID 17689148 .
- ↑ S. Alotaibi et al .: Epstein-Barr virus in pediatric multiple sclerosis. In: JAMA. Volume 291, Number 15, April 2004, pp. 1875-1879, doi: 10.1001 / jama.291.15.1875 . PMID 15100207 .
- ↑ JF Kurtzke et al .: Multiple sclerosis in the Faroe Islands. 5. The occurrence of the fourth epidemic as validation of transmission. In: Acta neurologica Scandinavica. Volume 88, Number 3, September 1993, pp. 161-173, PMID 8256551 .
- ↑ JF Kurtzke, K. Hyllested: Multiple sclerosis in the Faroe Islands: I. Clinical and epidemiological features. In: Annals of neurology. Volume 5, Number 1, January 1979, pp. 6-21, doi: 10.1002 / ana.410050104 . PMID 371519 .
- ^ AL Ponsonby et al .: Exposure to infant siblings during early life and risk of multiple sclerosis. In: JAMA. Volume 293, Number 4, January 2005, pp. 463-469, doi: 10.1001 / jama.293.4.463 . PMID 15671431 .
- ↑ Christine Westerhaus: SCHWEINEPEITSCHENWURM - Helper against multiple sclerosis? , in Deutschlandfunk - “ Research News ” from January 24, 2014.
- ^ IA van der Mei et al .: Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. In: BMJ. Volume 327, number 7410, August 2003, p. 316, doi: 10.1136 / bmj.327.7410.316 . PMID 12907484 . PMC 169645 (free full text).
- ^ N. Kromann, A. Green: Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950-1974. In: Acta medica Scandinavica. Volume 208, Number 5, 1980, pp. 401-406, PMID 7457208 .
- ↑ B. Deutch et al .: Traditional and modern Greenlandic food - dietary composition, nutrients and contaminants. In: Science of the Total Environment . Volume 384, number 1-3, October 2007, pp. 106-119, doi: 10.1016 / j.scitotenv.2007.05.042 . PMID 17629548 .
- ↑ Kassandra L. Munger et al .: Dietary intake of vitamin D during adolescence and risk of multiple sclerosis . In: Journal of Neurology . tape 258 , no. 3 , March 2011, p. 479-485 , doi : 10.1007 / s00415-010-5783-1 , PMID 20945071 , PMC 3077931 (free full text).
- ↑ MY Hachim, NM Elemam, AA Maghazachi: The Beneficial and Debilitating Effects of Environmental and Microbial Toxins, Drugs, Organic Solvents and Heavy Metals on the onset and progression of Multiple Sclerosis . In: Toxins . tape 11 , no. 3 , March 2019, doi : 10.3390 / toxins11030147 , PMID 30841532 , PMC 6468554 (free full text).
- ↑ KK Aminzadeh, M. Etminan: Dental amalgam and multiple sclerosis: a systematic review and meta-analysis. In: Journal of public health dentistry. Volume 67, Number 1, 2007, pp. 64-66, PMID 17436982 . (Meta-analysis).
- ^ CH Hawkes: Smoking is a risk factor for multiple sclerosis: a metanalysis. In: Multiple sclerosis. Volume 13, Number 5, June 2007, pp. 610-615, doi: 10.1177 / 1352458506073501 . PMID 17548439 .
- ↑ T. Riise, MW Nortvedt, A. Ascherio: Smoking is a risk factor for multiple sclerosis. In: Neurology. Volume 61, Number 8, October 2003, pp. 1122-1124, PMID 14581676 .
- ↑ F. di Pauli et al .: Smoking is a risk factor for early conversion to clinically definite multiple sclerosis. In: Multiple sclerosis. Volume 14, Number 8, September 2008, pp. 1026-1030, doi: 10.1177 / 1352458508093679 . PMID 18632775 .
- ↑ P. Sundström, L. Nyström: Smoking words the prognosis in multiple sclerosis. In: Multiple sclerosis. Volume 14, Number 8, September 2008, pp. 1031-1035, doi: 10.1177 / 1352458508093615 . PMID 18632778 .
- ↑ K. Konieczka, S. Koch, T. Binggeli, A. Schoetzau, J. Kesselring: Multiple sclerosis and primary vascular dysregulation (Flammer syndrome). EPMA Journal 2016 Jun 15; 7:13
- ↑ MA Hernán et al .: Recombinant hepatitis B vaccine and the risk of multiple sclerosis: a prospective study. In: Neurology. Volume 63, Number 5, September 2004, pp. 838-842, PMID 15365133 .
- ↑ Y. Mikaeloff et al .: Hepatitis B vaccination and the risk of childhood-onset multiple sclerosis. In: Archives of pediatrics & adolescent medicine. Volume 161, number 12, December 2007, pp. 1176-1182, doi: 10.1001 / archpedi.161.12.1176 . PMID 18056563 .
- ↑ F. DeStefano et al .: Vaccinations and risk of central nervous system demyelinating diseases in adults. In: Archives of Neurology Volume 60, Number 4, April 2003, pp. 504-509, doi: 10.1001 / archneur.60.4.504 . PMID 12707063 .
- ↑ Y. Mikaeloff et al .: Hepatitis B vaccine and risk of relapse after a first childhood episode of CNS inflammatory demyelination. In: Brain. Volume 130, Pt 4 April 2007, pp. 1105-1110, doi: 10.1093 / brain / awl368 . PMID 17276994 .
- ↑ F. Zipp, JG Weil, KM Einhäupl: No increase in demyelinating diseases after hepatitis B vaccination. In: Nature medicine Volume 5, Number 9, September 1999, pp. 964-965, doi: 10.1038 / 12376 . PMID 10470051 .
- ^ Susanne Gallus: Vaccination does not cause multiple sclerosis. Medical Tribune , September 23, 2019, accessed October 15, 2019 .
- ↑ T. Putnam: "Encephalitis" and sclerotic plaques produced by venular obstruction. In: Arch Neurol Psychiatry , 1935; 33 (5), pp. 929-940.
- ^ T. Putnam: Evidences of vascular occlusion in multiple sclerosis and encephalomyelitis. In: Arch Neurol Psychiatry. 1937; 37 (6), pp. 1298-1321.
- ↑ T. Putnam, A. Adler: Vascular architecture of the lesions of multiple sclerosis. In: Arch Neurol Psychiat. 38: 1, 1937.
- ↑ RS Dow, G. Berglund: Vascular pattern of lesions of multiple sclerosis. In: Arch Neurol Psychiatry. 1942; 47 (1), pp. 1-18.
- ↑ F. Schelling: Damaging venous reflux into the skull or spine: relevance to multiple sclerosis. In: Medical hypotheses. Volume 21, Number 2, October 1986, pp. 141-148, PMID 3641027 .
- ↑ P. Zamboni et al .: Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. In: Journal of neurology, neurosurgery, and psychiatry . Volume 80, number 4, April 2009, pp. 392-399, doi: 10.1136 / jnnp.2008.157164 . PMID 19060024 . PMC 2647682 (free full text).
- ↑ M. Simka: Blood brain barrier compromise with endothelial inflammation may lead to autoimmune loss of myelin during multiple sclerosis. In: Current neurovascular research. Volume 6, Number 2, May 2009, pp. 132-139, PMID 19442163 . (Review article).
- ↑ German Multiple Sclerosis Society: Is an impaired blood flow in the brain due to venous insufficiency (CCSVI) the cause of MS? ( Memento of December 14, 2009 in the Internet Archive ) Retrieved November 8, 2009.
- ↑ P. Sundström et al .: Venous and cerebrospinal fluid flow in multiple sclerosis: a case-control study. In: Annals of neurology. Volume 68, Number 2, August 2010, pp. 255-259, doi: 10.1002 / ana.22132 . PMID 20695018 .
- ↑ F. Doepp et al .: No cerebrocervical venous congestion in patients with multiple sclerosis. In: Annals of neurology. Volume 68, Number 2, August 2010, pp. 173-183, doi: 10.1002 / ana.22085 . PMID 20695010 .
- ^ BA Bagert, E. Marder, O. Stüve: Chronic cerebrospinal venous insufficiency and multiple sclerosis. In: Archives of neurology. Volume 68, number 11, November 2011, pp. 1379-1384, doi: 10.1001 / archneurol.2011.179 . PMID 21747006 . (Review article).
- ↑ Neurologists warn against dangerous therapy for multiple sclerosis. (PDF) DGN, September 17, 2010, accessed on September 22, 2010 .
- ↑ AL Traboulsee et al .: Prevalence of extracranial venous narrowing on catheter venography in people with multiple sclerosis, Their siblings, and unrelated healthy controls: a blinded, case-control study. In: The Lancet. 2013 Oct 8. pii: S0140-6736 (13) 61747-X doi: 10.1016 / S0140-6736 (13) 61747-X , PMID 24119384 .
- ^ R. Gold et al .: Overview and analysis of international case-control studies on "Chronic cerebrospinal venous insufficiency" (CCSVI) and multiple sclerosis. In: Current Neurology. 2013; 40 (08), pp. 445-451. doi: 10.1055 / s-0033-1351271
- ↑ H. Lassmann: Experimental models of multiple sclerosis. In: Revue neurologique. Volume 163, Numbers 6-7, June 2007, pp. 651-655, PMID 17607184 . (Review article).
- ^ PP Ho et al .: Identification of Naturally Occurring Fatty Acids of the Myelin Sheath That Resolve Neuroinflammation. In: Science Translational Medicine. 4, 2012, pp. 137ra73-137ra73, doi: 10.1126 / scitranslmed.3003831 .
- ^ Moises Velasquez-Manoff: Gut Microbiome: The Peacekeepers . In: Nature . 2015. doi : 10.1038 / 518S3a .
- ↑ Intestinal bacteria ensure a healthy brain On: Press release of the University Clinic Freiburg from June 1, 2015.
- ↑ Could Multiple Sclerosis Begin in the Gut? On: Scientific American dated October 8, 2014.
- ↑ James D. Thacker: The law of unintended consequences and antibiotics . In: Open Journal of Immunology . 02, No. 2, 2012, p. 59. doi : 10.4236 / oji.2012.22007 .
- ↑ Anjelique Schulfer, Martin J. Blaser: Risks of Antibiotic exposure Early in Life on the Developing microbiome . In: PLoS Pathogens . 2015. doi : 10.1371 / journal.ppat.1004903 .
- ↑ Cell , Vol. 180, issue 6, p. 1067-1080 (2020), DOI: https://doi.org/10.1016/j.cell.2020.02.035
- ^ FD Lublin, SC Reingold: Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. In: Neurology. Volume 46, Number 4, April 1996, pp. 907-911, PMID 8780061 .
- ↑ BG Weinshenker et al .: The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. In: Brain. Volume 112 (Pt 1), February 1989, pp. 133-146, PMID 2917275 .
- ↑ D. Buljevac et al .: Prospective study on the relationship between infections and multiple sclerosis exacerbations. In: Brain. Volume 125, Pt 5 May 2002, pp. 952-960, PMID 11960885 .
- ↑ C. Confavreux: Infections and the risk of relapse in multiple sclerosis. In: Brain. Volume 125, Pt 5 May 2002, pp. 933-934, PMID 11960883 .
- ^ S. Vukusic et al., The Pregnancy In Multiple Sclerosis Group: Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. In: Brain. Volume 127, Pt 6 June 2004, pp. 1353-1360, doi: 10.1093 / brain / awh152 . PMID 15130950 .
- ^ DS Goodin et al .: The relationship of MS to physical trauma and psychological stress: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. In: Neurology. Vol. 52, Number 9, June 1999, pp. 1737-1745, PMID 10371517 .
- ^ DC Mohr et al ..: Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. In: BMJ. Volume 328, number 7442, March 2004, p. 731, doi: 10.1136 / bmj.38041.724421.55 . PMID 15033880 . PMC 381319 (free full text). (Review article).
- ↑ A. Apel et al .: In: Advances in Neurology-Psychiatry. Volume 74, Number 10, October 2006, pp. 567-574, doi: 10.1055 / s-2005-919090 . PMID 16586258 . (Review article).
- ^ AJ Thompson et al .: Primary progressive multiple sclerosis. In: Brain. Volume 120 (Pt 6), June 1997, pp. 1085-1096, PMID 9217691 . (Review article).
- ↑ JM Dargin, RA Lowenstein: The painful eye. In: Emergency medicine clinics of North America. Volume 26, Number 1, February 2008, pp. 199-216, viii, doi: 10.1016 / j.emc.2007.10.001 . PMID 18249263 . (Review article).
- ↑ C. Solaro et al .: The prevalence of pain in multiple sclerosis: a multicenter cross-sectional study. In: Neurology. Volume 63, Number 5, September 2004, pp. 919-921, PMID 15365151 .
- ^ O. Ghaffar, A. Feinstein: The neuropsychiatry of multiple sclerosis: a review of recent developments. In: Current opinion in psychiatry. Volume 20, Number 3, May 2007, pp. 278-285, doi: 10.1097 / YCO.0b013e3280eb10d7 . PMID 17415083 . (Review article).
- ↑ SM Rao: Multiple sclerosis. In: JL Cummings (Ed.): Subcortical dementia . Oxford University Press, New York 1990, pp. 164-180.
- ↑ WI McDonald et al .: Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. In: Annals of neurology. Volume 50, Number 1, July 2001, pp. 121-127, PMID 11456302 .
- ^ A b Polman et al .: Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". In: Annals of neurology. Volume 58, Number 6, December 2005, pp. 840-846, doi: 10.1002 / ana.20703 . PMID 16283615 . (Review article).
- ↑ Overview of the changes to the revised version of the Mc-Donald criteria at the DMSG ( Memento of October 13, 2007 in the Internet Archive )
- ↑ Polman et al .: Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. In: Annals of neurology. Volume 69, number 2, February 2011, pp. 292-302, doi: 10.1002 / ana.22366 . PMID 21387374 . PMC 3084507 (free full text).
- ↑ Giovannoni et al .: Serum inflammatory markers and clinical / MRI markers of disease progression in multiple sclerosis. In: Journal of neurology. Volume 248, Number 6, June 2001, pp. 487-495, PMID 11499639 .
- ↑ Berger et al .: Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. In: The New England Journal of Medicine . Volume 349, Number 2, July 2003, pp. 139-145, doi: 10.1056 / NEJMoa022328 . PMID 12853586 .
- ↑ Kuhle et al .: Lack of association between antimyelin antibodies and progression to multiple sclerosis. In: The New England Journal of Medicine . Volume 356, Number 4, January 2007, pp. 371-378, doi: 10.1056 / NEJMoa063602 . PMID 17251533 .
- ↑ H. Link, YM Huang: Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. In: Journal of neuroimmunology. Volume 180, number 1–2, November 2006, pp. 17–28, doi: 10.1016 / j.jneuroim 2006.07.006 . PMID 16945427 . (Review article).
- ↑ H. Reiber, p Ungefehr, C. Jacobi: The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. In: Multiple sclerosis. Volume 4, Number 3, June 1998, pp. 111-117, PMID 9762657 .
- ↑ a b c S. Jarius, P. Eichhorn, D. Franciotta, HF Petereit, G. Akman-Demir, M. Wick, B. Wildemann. The MRZ reaction as a highly specific marker of multiple sclerosis: re-evaluation and structured review of the literature. In: J Neurol. , 2016, doi: 10.1007 / s00415-016-8360-4 , PMID 28005176
- ↑ LA rolák, JO Fleming: The differential diagnosis of multiple sclerosis. In: The neurologist. Volume 13, Number 2, March 2007, pp. 57-72, doi: 10.1097 / 01.nrl.0000254705.39956.34 . PMID 17351525 . (Review article).
- ↑ BG Weinshenker et al .: A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. In: Annals of neurology. Volume 46, Number 6, December 1999, pp. 878-886, PMID 10589540 .
- ^ DM Sutton et al .: Complications of plasma exchange. In: transfusion. Volume 29, Number 2, February 1989, pp. 124-127, PMID 2919422 .
- ^ MH Mokrzycki, AA Kaplan: Therapeutic plasma exchange: complications and management. In: American journal of kidney diseases. Volume 23, Number 6, June 1994, pp. 817-827, PMID 8203364 .
- ↑ Gregoriadis et al. Considerations on long-term immuno-intervention in the treatment of multiple sclerosis: an expert opinion. Expert Opin Pharmacother. 2016 Oct; 17 (15): 2085-95; doi: 10.1080 / 14656566.2016.1232712 . PMID 27594523 .
- ↑ HL Weiner et al. Immunosuppressive treatment in multiple sclerosis. J Neurol Sci. 2004 Aug 15; 223 (1): 1-11. doi: 10.1016 / j.jns.2004.04.013 PMID 15261554 .
- ↑ Schreier et al .: Infections in the Immunocompromised Host.
- ↑ Oh and O'Connor. Established disease-modifying treatments in relapsing-remitting multiple sclerosis. 2015 Jun; 28 (3): 220-9. doi: 10.1097 / WCO.0000000000000202 . PMID 25923124 .
- ↑ Penn et Starzl. Malignant tumors arising de novo in immunosuppressed organ transplant recipients. 1972 Oct; 14 (4): 407-17. PMC 3006181 (free full text). PMID 4345337 .
- ↑ Ghezzi et al. Disease reactivation after fingolimod discontinuation in two multiple sclerosis patients. 2013 Jan; 260 (1): 327-9. doi: 10.1007 / s00415-012-6744-7 - PMID 23161460 .
- ^ German Society for Neurology. Guidelines for Diagnostics and Therapy in Neurology (PDF)
- ↑ Diagnosis and therapy of multiple sclerosis Updated guidelines of the DGN, accessed on March 16, 2019
- ↑ Cell , Vol. 180, issue 6, p. 1067-1080 (2020), DOI: https://doi.org/10.1016/j.cell.2020.02.035
- ↑ Quality Manual MS / NMOSD Recommendations for the Therapy of Multiple Sclerosis / Neuro myelitis optica spectrum diseases for doctors , 3rd revised and extended edition July 2018, publisher: Disease-related Competence Network Multiple Sklerose e. V
- ^ Peter Rieckmann, Hans-Peter Hartung, Klaus V. Toyka: Multiple Sclerosis Recombinant Beta-Interferons: Immunomodulatory Therapy of Relapsing Multiple Sclerosis . In: Deutsches Ärzteblatt . tape 93 , no. 46 . Deutscher Ärzte-Verlag, November 15, 1996, p. A-3022 / B-2553 / C-2272 ( aerzteblatt.de ).
- ↑ Filippini et al .: Interferons in relapsing remitting multiple sclerosis: a systematic review. In: Lancet. Volume 361, Number 9357, February 2003, pp. 545-552, doi: 10.1016 / S0140-6736 (03) 12512-3 . PMID 12598138 . (Meta-analysis).
- ↑ R. Gold: Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis . In: N Engl J Med . tape 367 , September 2012, p. 1098-1107 , doi : 10.1056 / NEJMoa1114287 .
- ↑ RJ Fox: Placebo-Controlled Phase 3 Study of Oral BG-12 or Glatiramer in Multiple Sclerosis . In: N Engl J Med . tape 367 , September 2012, p. 1087-1097 , doi : 10.1056 / NEJMoa1206328 .
- ↑ L. Munari, R. Lovati, A. Boiko: Therapy with glatiramer acetate for multiple sclerosis. In: Cochrane database of systematic reviews (online). Number 1, 2004, S. CD004678, doi: 10.1002 / 14651858.CD004678 . PMID 14974077 . (Meta-analysis).
- ^ I. Casetta, G. Iuliano, G. Filippini: Azathioprine for multiple sclerosis. In: Cochrane database of systematic reviews (online). Number 4, 2007, S. CD003982, doi: 10.1002 / 14651858.CD003982.pub2 . PMID 17943809 . (Meta-analysis).
- ^ O. Gray, GV McDonnell, RB Forbes: Intravenous immunoglobulins for multiple sclerosis. In: Cochrane database of systematic reviews (online). Number 4, 2003, S. CD002936, doi: 10.1002 / 14651858.CD002936 . PMID 14583956 . (Meta-analysis).
- ↑ JA Cohen et al .: Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. In: The New England Journal of Medicine . Volume 362, Number 5, February 2010, pp. 402-415, doi: 10.1056 / NEJMoa0907839 . PMID 20089954 .
- ↑ L. Kappos et al .: A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. In: The New England Journal of Medicine . Volume 362, Number 5, February 2010, pp. 387-401, doi: 10.1056 / NEJMoa0909494 . PMID 20089952 .
- ↑ Polman et al .: A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. In: The New England Journal of Medicine . Volume 354, Number 9, March 2006, pp. 899-910, doi: 10.1056 / NEJMoa044397 . PMID 16510744 .
- ↑ Martinelli Boneschi et al .: Mitoxantrone for multiple sclerosis. In: Cochrane database of systematic reviews (online). Number 4, 2005, S. CD002127, doi: 10.1002 / 14651858.CD002127.pub2 . PMID 16235298 . (Meta-analysis).
- ↑ La Mantia et al .: Cyclophosphamide for multiple sclerosis. In: Cochrane database of systematic reviews (online). Number 1, 2007, S. CD002819, doi: 10.1002 / 14651858.CD002819.pub2 . PMID 17253481 . (Meta-analysis).
- ↑ CHMP recommends amendment of the approval of Rebif® for the clinically necessary use during pregnancy and breastfeeding , PM Merck of September 23, 2019, accessed on October 28, 2019
- ↑ Summary of Product Characteristics EMA approval text using Rebif as an example, accessed on October 28, 2019
- ↑ De Jong et al .: Confusing Cochrane reviews on treatment in multiple sclerosis. In: Lancet neurology. Volume 4, Number 6, June 2005, pp. 330-331, doi: 10.1016 / S1474-4422 (05) 70081-8 . PMID 15907736 . (Comment)
- ^ European Medicines Agency - Find medicine - Mavenclad. Accessed August 31, 2018 .
- ^ Office of the Commissioner: Press Announcements - FDA approves new oral treatment for multiple sclerosis. Retrieved March 30, 2019 .
- ↑ Biogen. Biogen and AbbVie's Once-Monthly ZINBRYTA ™ (daclizumab) Approved in European Union for Treatment of Multiple Sclerosis ( Memento from February 17, 2017 in the Internet Archive ).
- ↑ Fingolimod (Gilenya®): First report of progressive multifocal leukoencephalopathy (PML) in a multiple sclerosis patient on fingolimod therapy without prior treatment with natalizumab or other immunosuppressive drugs RHB dated May 4, 2015, accessed on March 16, 2019
- ↑ Fingolimod (Gilenya®): Risks Associated with Effects on the Immune System , RHB January 2016, accessed March 16, 2019
- ↑ Fingolimod (Gilenya®): Contraindications for patients with cardiac diseases , RHB of November 6, 2017, accessed on March 16, 2019
- ↑ Fingolimod (Gilenya®): New contraindication for use during pregnancy and in women of childbearing age who are not using an effective method of contraception , RHB of September 2, 2019, accessed on February 26, 2020
- ↑ Use of multiple sclerosis medicine Lemtrada restricted while EMA review is ongoing , PM EMA of April 12, 2019, accessed on February 12, 2020
- ↑ Alemtuzumab - only for severe cases , Pharmazeutische Zeitung of April 12, 2019, accessed on February 12, 2020
- ↑ Alemtuzumab (LEMTRADA): Restriction of use in multiple sclerosis due to safety concerns , Red Hand Brief Sanofi April 2019, accessed on February 12, 2020
- ↑ LEMTRADA (alemtuzumab): restriction of the indication, additional contraindications and risk-minimizing measures , Red Hand Brief Sanofi January 2020, accessed on February 12, 2020
- ↑ Updated safety of cladribine tablets in the treatment of patients with multiple sclerosis: Integrated safety analysis and post-approval data , ECTRIMS Kongress, Stockholm September 2019, accessed on February 12, 2020
- ↑ Ron Winslow: After 40-year odyssey, first drug for aggressive MS wins FDA approval , STAT. March 28, 2017. Archived from the original on April 1, 2017.
- ^ Ocrelizumab label . FDA. March 2017. Archived from the original on April 1, 2017. Retrieved April 1, 2017. See also FDA index page for BLA 761053 for updates; April 1, 2017 ( Memento from April 1, 2017 in the Internet Archive )
- ^ BLA Approval Letter . FDA. March 28, 2017. Archived from the original on April 2, 2017.
- ↑ Switzerland allows MS antibody Ocrevus . Retrieved October 4, 2017.
- ^ John Miller, Michael Shields: Roche's star MS medicine Ocrevus wins EU approval. In: Reuters. January 12, 2018, accessed January 12, 2018 .
- ↑ Katharine Harding et al .: Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients With Multiple Sclerosis In: JAMA Neurol , February 2019, pp. 643–652, doi: 10.1001 / jamaneurol.2018.4905 . (Original work).
- ↑ Use the time window! , Comment in Ärzte Zeitung online, February 24, 2019
- ↑ Which therapy might be best for you , a guide
- ↑ Celgene Submits Application to EMA for Ozanimod for the Treatment of Relapsing-Remitting Multiple Sclerosis , Celgene PM, March 11, 2019, accessed March 16, 2019
- ↑ Zeposia , EMA, positive opinion, accessed April 10, 2020
- ↑ Novartis announces positive phase III results showing efficacy of BAF312 in patients with secondary progressive MS , PM Novartis from August 25, 2016
- ↑ EPAR of the EMA , SUMMARY OF THE CHARACTERISTICS OF THE MEDICINAL PRODUCT on the EMA website, accessed on February 26, 2020
- ↑ Merck with the very latest positive phase II data on the oral test therapy evobrutinib at RMS ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. , Merck PM on October 12, 2018, accessed March 17, 2019
- ↑ MB Rietberg et al .: Exercise therapy for multiple sclerosis. In: Cochrane database of systematic reviews (online). Number 1, 2005, S. CD003980, doi: 10.1002 / 14651858.CD003980.pub2 . PMID 15674920 . (Meta-analysis)
- ↑ J. Kesselring, S. Beer: Symptomatic therapy and neurorehabilitation in multiple sclerosis. In: Lancet neurology. Volume 4, Number 10, October 2005, pp. 643-652, doi: 10.1016 / S1474-4422 (05) 70193-9 . PMID 16168933 . (Review article).
- ↑ Summary of the EPAR for the public (PDF) from the website of the European Health Authority (EMA), accessed on February 10, 2014.
- ↑ DT Shakespeare, M. Boggild, C. Young: Anti-spasticity agents for multiple sclerosis. In: Cochrane database of systematic reviews (online). Number 4, 2003, S. CD001332, doi: 10.1002 / 14651858.CD001332 . PMID 14583932 .
- ↑ Tetrahydrocannabinol and Cannabidiol – Sativex ® –05–2011. In: Pharmaceutical newspaper online. 2011, accessed December 27, 2014 .
- ↑ Drugs Directive / Annex VI: Gabapentin for the treatment of spasticity in the context of multiple sclerosis , G-BA decision of March 28, 2014, accessed on March 29, 2014.
- ↑ Pöllmann et al .: Therapy of pain in MS - an overview with evidence-based therapy recommendations. In: Advances in neurological psychiatry. Volume 73, Number 5, May 2005, pp. 268-285, doi: 10.1055 / s-2004-830193 . PMID 15880305 . (Review article).
- ↑ V. Kalsi, CJ Fowler: Therapy Insight: bladder dysfunction associated with multiple sclerosis. In: Nature clinical practice. Urology. Volume 2, number 10, October 2005, pp. 492-501, doi: 10.1038 / ncpuro0323 . PMID 16474623 . (Review article).
- ↑ RM Merson, MI Rolnick: Speech-language pathology and dysphagia in multiple sclerosis. In: Physical medicine and rehabilitation clinics of North America. Volume 9, Number 3, August 1998, pp. 631-641, PMID 9894114 . (Review article).
- ^ Psychological interventions for multiple sclerosis. In: Cochrane database of systematic reviews (online). Number 1, 2006, S. CD004431, doi: 10.1002 / 14651858.CD004431.pub2 . PMID 16437487 .
- ↑ Tomassini et al .: Comparison of the effects of acetyl L-carnitine and amantadine for the treatment of fatigue in multiple sclerosis: results of a pilot, randomized, double-blind, crossover trial. In: Journal of the neurological sciences. Volume 218, number 1-2, March 2004, pp. 103-108, doi: 10.1016 / j.jns.2003.11.005 . PMID 14759641 .
- ↑ Wingerchuk et al .: A randomized controlled crossover trial of aspirin for fatigue in multiple sclerosis. In: Neurology. Volume 64, number 7, April 2005, pp. 1267-1269, doi: 10.1212 / 01.WNL.0000156803.23698.9A . PMID 15824361 .
- ↑ Rammohan et al .: Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two center phase 2 study. In: J Neurol Neurosurg Psychiatry. 2002; 72, pp. 179-183 PMID 11796766
- ↑ Pucci et al .: Amantadine for fatigue in multiple sclerosis. In: Cochrane database of systematic reviews (online). Number 1, 2007, S. CD002818, doi: 10.1002 / 14651858.CD002818.pub2 . PMID 17253480 . (Meta-analysis).
- ↑ Stankoff et al .: Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. In: Neurology. Volume 64, number 7, April 2005, pp. 1139-1143, doi: 10.1212 / 01.WNL.0000158272.27070.6A . PMID 15824337 .
- ↑ BM Hulter, PO Lundberg: Sexual function in women with advanced multiple sclerosis. In: Journal of neurology, neurosurgery, and psychiatry . Volume 59, Number 1, July 1995, pp. 83-86, PMID 7608715 . PMC 1073606 (free full text).
- ↑ M. Zorzon et al .: Sexual dysfunction in multiple sclerosis: a case-control study. I. Frequency and comparison of groups. In: Multiple sclerosis. Volume 5, Number 6, December 1999, pp. 418-427, PMID 10618699 .
- ↑ D. Borello-France et al .: Bladder and sexual function among women with multiple sclerosis. In: Multiple sclerosis. Volume 10, Number 4, August 2004, pp. 455-461, PMID 15327046 .
- ↑ R. DasGupta, CJ Fowler: sexual and urological dysfunction in multiple sclerosis: better understanding and improved therapies. In: Current opinion in neurology. Volume 15, Number 3, June 2002, pp. 271-278, PMID 12045724 . (Review).
- ↑ M. Farinotti et al .: Dietary interventions for multiple sclerosis. In: Cochrane database of systematic reviews. Volume 12, 2012, pp. CD004192, doi: 10.1002 / 14651858.CD004192.pub3 . PMID 23235605 . (Meta-analysis)
- ↑ Apel et al .: Frequency of current utilization of complementary and alternative medicine by patients with multiple sclerosis. In: Journal of neurology. Volume 253, Number 10, October 2006, pp. 1331-1336, doi: 10.1007 / s00415-006-0217-9 . PMID 16786211 .
- ↑ Pucci et al .: Why physicians need to look more closely at the use of complementary and alternative medicine by multiple sclerosis patients. In: European journal of neurology. Volume 11, Number 4, April 2004, pp. 263-267, doi: 10.1046 / j.1468-1331.2003.00758.x . PMID 15061828 .
- ↑ S. Schwarz et al .: Alternative and complementary therapies for multiple sclerosis. In: Advances in neurological psychiatry. Volume 73, Number 8, August 2005, pp. 451-462, doi: 10.1055 / s-2004-830248 . PMID 16052439 . (Review article)
- ↑ Query at Clinicaltrials.org on November 12, 2013.
- ^ BA Cohen, P. Rieckmann: Emerging oral therapies for multiple sclerosis. In: International journal of clinical practice. Volume 61, number 11, November 2007, pp. 1922-1930, doi: 10.1111 / j.1742-1241.2007.01561..x . PMID 17784852 . (Review).
- ↑ PA Muraro, B. Bielekova: Emerging therapies for multiple sclerosis. In: Neurotherapeutics. Volume 4, number 4, October 2007, pp. 676-692, doi: 10.1016 / j.nurt.2007.07.003 . PMID 17920549 . (Review of current research focuses)
- ↑ Christoph Kleinschnitz et al .: Multiple sclerosis therapy: an update on recently finished trials. In: Journal of neurology. Volume 254, Number 11, November 2007, pp. 1473-1490, doi: 10.1007 / s00415-007-0684-7 . PMID 18004638 . (Review). (Review of current research focuses)
- ↑ Blanco et al .: Autologous haematopoietic-stem-cell transplantation for multiple sclerosis. In: Lancet neurology. Volume 4, number 1, January 2005, pp. 54-63, doi: 10.1016 / S1474-4422 (04) 00966-4 . PMID 15620857 . (Review article on autologous stem cell transplantation)
- ↑ Gladstone et al .: High-dose cyclophosphamide for moderate to severe refractory multiple sclerosis. In: Archives of neurology. Volume 63, Number 10, October 2006, pp. 1388-1393, doi: 10.1001 / archneur.63.10.noc60076 . PMID 16908728 .
- ↑ HL Atkins et al .: Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. June 8, 2016, pii: S0140-6736 (16) 30169-6. PMID 27291994 , doi: 10.1016 / S0140-6736 (16) 30169-6 .
- ↑ Vana et al .: Platelet-derived growth factor promotes repair of chronically demyelinated white matter. In: Journal of Neuropathology & Experimental Neurology , Volume 66, Number 11, November 2007, pp. 975-988, doi: 10.1097 / NEN.0b013e3181587d46 . PMID 17984680 . PMC 2788485 (free full text).
- ↑ Nait-Oumesmar et al .: Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. In: Proceedings of the National Academy of Sciences . Volume 104, Number 11, March 2007, pp. 4694-4699, doi: 10.1073 / pnas.0606835104 . PMID 17360586 . PMC 3025281 (free full text).
- ↑ Full text 'MS in focus' 11/2008 Stem Cells and Remylination in MS ( Memento from June 25, 2012 in the Internet Archive ) (PDF; 1.4 MB)
- ^ G. Ristori et al .: Effects of Bacille Calmette-Guerin after the first demyelinating event in the CNS. In: Neurology. doi: 10.1212 / 01.wnl.0000438216.93319.ab .
- ^ CM Rice et al .: Stem Cells 1: Cell therapy for multiple sclerosis: an evolving concept with implications for other neuro-degenerative diseases. Lancet 2013; 382, pp. 1204-1213.
- ^ Pittock et al .: Change in MS-related disability in a population-based cohort: a 10-year follow-up study. In: Neurology. Volume 62, Number 1, January 2004, pp. 51-59, PMID 14718697 .
- ↑ Daumer et al .: Prognosis of the individual course of disease-steps in developing a decision support tool for multiple sclerosis. In: BMC medical informatics and decision making. Volume 7, 2007, p. 11, doi: 10.1186 / 1472-6947-7-11 . PMID 17488517 . PMC 1884137 (free full text). There imagined Web-based program to determine the risk profile .
- ^ S2 guideline: Diagnosis and therapy of multiple sclerosis. Status January 2012 (supplement August 2014) , Working Group of Scientific Medical Societies, accessed on October 28, 2019.
- ↑ Disease-related Competence Network Multiple Sclerosis e. V. (Ed.): Quality Manual MS / NMOSD. Recommendations for the therapy of multiple sclerosis / neuromyelitis optica spectrum diseases for doctors. 3rd revised and expanded edition July 2018; Page 244
- ↑ Rate of Pregnancy-Related Relapse in Multiple Sclerosis , NEJM doi: 10.1056 / NEJM199807303390501 , accessed October 28, 2019.
- ↑ Berger T, Di Pauli F. J Neurol Neurochir Psychiatr 2009; 10: 26-31
- ↑ a b Hellwig K et al. Ther Adv Neurol Disord 2012; 5: 247-53
- ↑ a b c d e f g h EMA -> "Medicines" -> "Search": Enter the name of the drug and click search - scroll down and find the article with the name of the drug and "European public assessment report (EPAR)" in the title click on - Scroll down this page again and click on "Product information" -> select the language "German" and scroll to the "Pregnancy, lactation, fertility" section.
- ↑ Patient information on treatment with mitoxantrone , Competence Network Multiple Sclerosis, accessed on July 26, 2020
- ^ Technical information Copaxone , website of the manufacturer, accessed on July 26, 2020
- ↑ Fachinfo Clift , website of the manufacturer, accessed on July 26, 2020
- ↑ DKMS German bone marrow donor file (accessed on May 19, 2010)
- ^ Statement of the Medical Advisory Board of the DMSG, Bundesverband e. V. on organ transplantation, blood donation and stem cell donation in multiple sclerosis. ( Memento from January 16, 2013 in the Internet Archive ) German Multiple Sclerosis Society, November 13, 2012; Retrieved February 28, 2013.
- ↑ 90-second version


