Granulocyte-macrophage colony-stimulating factor: Difference between revisions
PubMed Central ID was added |
Citation bot (talk | contribs) Alter: volume. Add: doi-access. | Use this bot. Report bugs. | Suggested by Headbomb | Linked from Wikipedia:WikiProject_Academic_Journals/Journals_cited_by_Wikipedia/Sandbox3 | #UCB_webform_linked 802/1975 |
||
| (12 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Mammalian protein found in Homo sapiens}} |
|||
{{Distinguish|granulocyte colony-stimulating factor|macrophage colony-stimulating factor}} |
{{Distinguish|granulocyte colony-stimulating factor|macrophage colony-stimulating factor}} |
||
{{Infobox_gene}} |
{{Infobox_gene}} |
||
| Line 5: | Line 6: | ||
| Name = Granulocyte-macrophage colony-stimulating factor |
| Name = Granulocyte-macrophage colony-stimulating factor |
||
| image = PDB 1csg EBI.jpg |
| image = PDB 1csg EBI.jpg |
||
| width = |
| width = |
||
| caption = three-dimensional structure of [[recombinant DNA|recombinant]] human granulocyte-macrophage colony-stimulating factor (rhGM_CSF) |
| caption = three-dimensional structure of [[recombinant DNA|recombinant]] human granulocyte-macrophage colony-stimulating factor (rhGM_CSF) |
||
| Pfam = PF01109 |
| Pfam = PF01109 |
||
| Pfam_clan = CL0053 |
| Pfam_clan = CL0053 |
||
| InterPro = IPR000773 |
| InterPro = IPR000773 |
||
| SMART = |
| SMART = |
||
| PROSITE = PDOC00584 |
| PROSITE = PDOC00584 |
||
| MEROPS = |
| MEROPS = |
||
| SCOP = 2gmf |
| SCOP = 2gmf |
||
| TCDB = |
| TCDB = |
||
| OPM family = |
| OPM family = |
||
| OPM protein = |
| OPM protein = |
||
| CAZy = |
| CAZy = |
||
| CDD = |
| CDD = |
||
}} |
}} |
||
{{Drugbox |
{{Drugbox |
||
| Line 42: | Line 43: | ||
'''Granulocyte-macrophage colony-stimulating factor''' ('''GM-CSF'''), also known as '''colony-stimulating factor 2 (CSF2)''', is a [[monomeric]] [[glycoprotein]] secreted by [[macrophage]]s, [[T cell]]s, [[mast cells]], [[natural killer cell]]s, [[endothelial cell]]s and [[fibroblast]]s that functions as a [[cytokine]]. The [[pharmaceutical drug|pharmaceutical]] analogs of naturally occurring GM-CSF are called [[sargramostim]] and [[molgramostim]]. |
'''Granulocyte-macrophage colony-stimulating factor''' ('''GM-CSF'''), also known as '''colony-stimulating factor 2 (CSF2)''', is a [[monomeric]] [[glycoprotein]] secreted by [[macrophage]]s, [[T cell]]s, [[mast cells]], [[natural killer cell]]s, [[endothelial cell]]s and [[fibroblast]]s that functions as a [[cytokine]]. The [[pharmaceutical drug|pharmaceutical]] analogs of naturally occurring GM-CSF are called [[sargramostim]] and [[molgramostim]]. |
||
Unlike [[granulocyte colony-stimulating factor]], which specifically promotes [[neutrophil]] proliferation and maturation, GM-CSF affects more cell types, especially macrophages and [[eosinophil]]s.<ref name="pmid10081506">{{cite journal | vauthors = Root RK, Dale DC | title = Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: comparisons and potential for use in the treatment of infections in nonneutropenic patients | journal = The Journal of Infectious Diseases | volume = 179 |
Unlike [[granulocyte colony-stimulating factor]], which specifically promotes [[neutrophil]] proliferation and maturation, GM-CSF affects more cell types, especially macrophages and [[eosinophil]]s.<ref name="pmid10081506">{{cite journal | vauthors = Root RK, Dale DC | title = Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: comparisons and potential for use in the treatment of infections in nonneutropenic patients | journal = The Journal of Infectious Diseases | volume = 179 | issue = Suppl 2 | pages = S342-52 | date = March 1999 | pmid = 10081506 | doi = 10.1086/513857 | doi-access = free }}</ref> |
||
== Function == |
== Function == |
||
GM-CSF is a [[monomeric]] [[glycoprotein]] that functions as a [[cytokine]] |
GM-CSF is a [[monomeric]] [[glycoprotein]] that functions as a [[cytokine]]—it is a [[white blood cell]] [[growth factor]].<ref name="pmid24264600">{{cite journal | vauthors = Francisco-Cruz A, Aguilar-Santelises M, Ramos-Espinosa O, Mata-Espinosa D, Marquina-Castillo B, Barrios-Payan J, Hernandez-Pando R | title = Granulocyte-macrophage colony-stimulating factor: not just another haematopoietic growth factor | journal = Medical Oncology | volume = 31 | issue = 1 | pages = 774 | date = January 2014 | pmid = 24264600 | doi = 10.1007/s12032-013-0774-6 | s2cid = 24452892 }}</ref> GM-CSF stimulates [[stem cell]]s to produce [[granulocyte]]s ([[neutrophil]]s, [[eosinophil]]s, and [[basophil]]s) and [[monocyte]]s. Monocytes exit the circulation and migrate into tissue, whereupon they mature into [[macrophage]]s and [[dendritic cell]]s. Thus, it is part of the [[immune system|immune]]/[[inflammation|inflammatory]] [[biochemical cascade|cascade]], by which activation of a small number of macrophages can rapidly lead to an increase in their numbers, a process crucial for fighting [[infection]].{{cn|date=April 2023}} |
||
GM-CSF also has some effects on mature cells of the immune system. These include, for example, enhancing neutrophil migration and causing an alteration of the receptors expressed on the cells surface.<ref>{{cite journal | vauthors = Gasson JC | title = Molecular physiology of granulocyte-macrophage colony-stimulating factor | journal = Blood | volume = 77 | issue = 6 | pages = 1131–45 | date = March 1991 | pmid = 2001448 | doi = 10.1182/blood.V77.6.1131.1131 | doi-access = free }}</ref> |
GM-CSF also has some effects on mature cells of the immune system. These include, for example, enhancing neutrophil migration and causing an alteration of the receptors expressed on the cells surface.<ref>{{cite journal | vauthors = Gasson JC | title = Molecular physiology of granulocyte-macrophage colony-stimulating factor | journal = Blood | volume = 77 | issue = 6 | pages = 1131–45 | date = March 1991 | pmid = 2001448 | doi = 10.1182/blood.V77.6.1131.1131 | doi-access = free }}</ref> |
||
GM-CSF signals via signal transducer and activator of transcription, [[STAT5]].<ref name="pmid23042651">{{cite journal | vauthors = Voehringer D | title = Basophil modulation by cytokine instruction | journal = European Journal of Immunology | volume = 42 | issue = 10 | pages = 2544–50 | date = October 2012 | pmid = 23042651 | doi = 10.1002/eji.201142318 }}</ref> In macrophages, it has also been shown to signal via [[STAT3]]. The cytokine activates macrophages to inhibit fungal survival. It induces deprivation in intracellular free zinc and increases production of [[reactive oxygen species]] that culminate in fungal zinc starvation and toxicity.<ref name="pmid24138881">{{cite journal | vauthors = Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS | title = Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival | journal = Immunity | volume = 39 | issue = 4 | pages = 697–710 | date = October 2013 | pmid = 24138881 | pmc = 3841917 | doi = 10.1016/j.immuni.2013.09.006 }}</ref> Thus, GM-CSF facilitates development of the immune system and promotes defense against infections. |
GM-CSF signals via signal transducer and activator of transcription, [[STAT5]].<ref name="pmid23042651">{{cite journal | vauthors = Voehringer D | title = Basophil modulation by cytokine instruction | journal = European Journal of Immunology | volume = 42 | issue = 10 | pages = 2544–50 | date = October 2012 | pmid = 23042651 | doi = 10.1002/eji.201142318 | s2cid = 23972211 | doi-access = free }}</ref> In macrophages, it has also been shown to signal via [[STAT3]]. The cytokine activates macrophages to inhibit fungal survival. It induces deprivation in intracellular free zinc and increases production of [[reactive oxygen species]] that culminate in fungal zinc starvation and toxicity.<ref name="pmid24138881">{{cite journal | vauthors = Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS | title = Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival | journal = Immunity | volume = 39 | issue = 4 | pages = 697–710 | date = October 2013 | pmid = 24138881 | pmc = 3841917 | doi = 10.1016/j.immuni.2013.09.006 }}</ref> Thus, GM-CSF facilitates development of the immune system and promotes defense against infections.{{cn|date=April 2023}} |
||
GM-CSF also plays a role in embryonic development by functioning as an [[embryokine]] produced by reproductive tract.<ref name="pmid24954585">{{cite journal | vauthors = Hansen PJ, Dobbs KB, Denicol AC | title = Programming of the preimplantation embryo by the embryokine colony stimulating factor 2 | journal = Animal Reproduction Science | volume = 149 | issue = |
GM-CSF also plays a role in embryonic development by functioning as an [[embryokine]] produced by reproductive tract.<ref name="pmid24954585">{{cite journal | vauthors = Hansen PJ, Dobbs KB, Denicol AC | title = Programming of the preimplantation embryo by the embryokine colony stimulating factor 2 | journal = Animal Reproduction Science | volume = 149 | issue = 1–2 | pages = 59–66 | date = September 2014 | pmid = 24954585 | doi = 10.1016/j.anireprosci.2014.05.017 }}</ref> |
||
== Genetics == |
== Genetics == |
||
| Line 60: | Line 61: | ||
== Glycosylation == |
== Glycosylation == |
||
Human granulocyte-macrophage colony-stimulating factor is glycosylated in its mature form. |
Human granulocyte-macrophage colony-stimulating factor is glycosylated in its mature form.{{cn|date=April 2023}} |
||
== History == |
== History == |
||
| Line 72: | Line 73: | ||
Sargramostim was approved by the US FDA in 1991 to accelerate white blood cell recovery following autologous [[bone marrow transplantation]] under the trade name Leukine, and passed through several hands, ending up with [[Genzyme]],<ref>{{cite web |url=http://www.pharmaceutical-technology.com/projects/berlex/ |title=Bayer Healthcare Pharmaceuticals Plant, Snohomish County, Washington State |publisher=pharmaceutical-technology.com |access-date=12 November 2011}}</ref> which was subsequently acquired by [[Sanofi]]. Leukine is now owned by Partner Therapeutics (PTx). |
Sargramostim was approved by the US FDA in 1991 to accelerate white blood cell recovery following autologous [[bone marrow transplantation]] under the trade name Leukine, and passed through several hands, ending up with [[Genzyme]],<ref>{{cite web |url=http://www.pharmaceutical-technology.com/projects/berlex/ |title=Bayer Healthcare Pharmaceuticals Plant, Snohomish County, Washington State |publisher=pharmaceutical-technology.com |access-date=12 November 2011}}</ref> which was subsequently acquired by [[Sanofi]]. Leukine is now owned by Partner Therapeutics (PTx). |
||
Imlygic was approved by the US FDA in October 2015,<ref>{{cite web |url=https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/imlygic-talimogene-laherparepvec |title=IMLYGIC (talimogene laherparepvec) |author= U.S. Food & Drug Administration |publisher=fda.gov |access-date=17 December 2019}}</ref> and in December 2015 by the EMA, as an oncolytic virotherapy, commercialized by Amgen Inc. This [[oncolytic herpes virus]], named [[Talimogene laherparepvec]], has been genetically engineered to express human GM-CSF using the tumor cells machinery.<ref>{{cite journal | vauthors = Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS | display-authors = 6 | title = Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma | journal = Journal of Clinical Oncology | volume = 33 | issue = 25 | pages = 2780–8 | date = September 2015 | pmid = 26014293 | doi = 10.1200/JCO.2014.58.3377 }}</ref> |
Imlygic was approved by the US FDA in October 2015,<ref>{{cite web |url=https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/imlygic-talimogene-laherparepvec |title=IMLYGIC (talimogene laherparepvec) |author= U.S. Food & Drug Administration |publisher=fda.gov |access-date=17 December 2019}}</ref> and in December 2015 by the EMA, as an oncolytic virotherapy, commercialized by Amgen Inc. This [[oncolytic herpes virus]], named [[Talimogene laherparepvec]], has been genetically engineered to express human GM-CSF using the tumor cells machinery.<ref>{{cite journal | vauthors = Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS | display-authors = 6 | title = Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma | journal = Journal of Clinical Oncology | volume = 33 | issue = 25 | pages = 2780–8 | date = September 2015 | pmid = 26014293 | doi = 10.1200/JCO.2014.58.3377 | doi-access = free }}</ref> |
||
==Clinical significance== |
==Clinical significance== |
||
GM-CSF is found in high levels in joints with [[rheumatoid arthritis]] and blocking GM-CSF as a [[biological target]] may reduce the inflammation or damage. Some drugs (e.g. [[otilimab]]) are being developed to ''block'' GM-CSF.<ref name="pmid23448220">{{cite journal | vauthors = Deiß A, Brecht I, Haarmann A, Buttmann M | title = Treating multiple sclerosis with monoclonal antibodies: a 2013 update | journal = Expert Review of Neurotherapeutics | volume = 13 | issue = 3 | pages = 313–35 | date = March 2013 | pmid = 23448220 | doi = 10.1586/ern.13.17 | s2cid = 169334 }}</ref> In critically ill patients GM-CSF has been trialled as a therapy for the immunosuppression of critical illness, and has shown promise restoring [[monocyte]]<ref>{{cite journal | vauthors = Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD | display-authors = 6 | title = Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial | journal = American Journal of Respiratory and Critical Care Medicine | volume = 180 | issue = 7 | pages = 640–8 | date = October 2009 | pmid = 19590022 | doi = 10.1164/rccm.200903-0363OC }}</ref> and [[neutrophil]]<ref>{{cite journal | vauthors = Pinder EM, Rostron AJ, Hellyer TP, Ruchaud-Sparagano MH, Scott J, Macfarlane JG, Wiscombe S, Widdrington JD, Roy AI, Linnett VC, Baudouin SV, Wright SE, Chadwick T, Fouweather T, Juss JK, Chilvers ER, Bowett SA, Parker J, McAuley DF, Conway Morris A, Simpson AJ | display-authors = 6 | title = Randomised controlled trial of GM-CSF in critically ill patients with impaired neutrophil phagocytosis | journal = Thorax | volume = 73 | issue = 10 | pages = 918–925 | date = October 2018 | pmid = 30064991 | pmc = 6166597 | doi = 10.1136/thoraxjnl-2017-211323 }}</ref> function, although the impact on patient outcomes is currently unclear and awaits larger studies. |
GM-CSF is found in high levels in joints with [[rheumatoid arthritis]] and blocking GM-CSF as a [[biological target]] may reduce the inflammation or damage. Some drugs (e.g. [[otilimab]]) are being developed to ''block'' GM-CSF.<ref name="pmid23448220">{{cite journal | vauthors = Deiß A, Brecht I, Haarmann A, Buttmann M | title = Treating multiple sclerosis with monoclonal antibodies: a 2013 update | journal = Expert Review of Neurotherapeutics | volume = 13 | issue = 3 | pages = 313–35 | date = March 2013 | pmid = 23448220 | doi = 10.1586/ern.13.17 | s2cid = 169334 }}</ref> In critically ill patients GM-CSF has been trialled as a therapy for the immunosuppression of critical illness, and has shown promise restoring [[monocyte]]<ref>{{cite journal | vauthors = Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD | display-authors = 6 | title = Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial | journal = American Journal of Respiratory and Critical Care Medicine | volume = 180 | issue = 7 | pages = 640–8 | date = October 2009 | pmid = 19590022 | doi = 10.1164/rccm.200903-0363OC }}</ref> and [[neutrophil]]<ref>{{cite journal | vauthors = Pinder EM, Rostron AJ, Hellyer TP, Ruchaud-Sparagano MH, Scott J, Macfarlane JG, Wiscombe S, Widdrington JD, Roy AI, Linnett VC, Baudouin SV, Wright SE, Chadwick T, Fouweather T, Juss JK, Chilvers ER, Bowett SA, Parker J, McAuley DF, Conway Morris A, Simpson AJ | display-authors = 6 | title = Randomised controlled trial of GM-CSF in critically ill patients with impaired neutrophil phagocytosis | journal = Thorax | volume = 73 | issue = 10 | pages = 918–925 | date = October 2018 | pmid = 30064991 | pmc = 6166597 | doi = 10.1136/thoraxjnl-2017-211323 }}</ref> function, although the impact on patient outcomes is currently unclear and awaits larger studies. |
||
GM-CSF stimulates [[monocyte]]s and [[macrophage]]s to produce pro-inflammatory cytokines, including [[CCL17]].<ref name="pmid33150139" /> Elevated GM-CSF has been shown to contribute to inflammation in [[inflammatory arthritis]], [[osteoarthritis]], [[colitis]] [[asthma]], [[obesity]], and [[Coronavirus disease 2019|COVID-19]].<ref name="pmid33150139" /><ref name=Thwaites>{{cite journal | vauthors = Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, Liew F, Russell CD, Moore SC, Fairfield C, Carter E, Abrams S, Short CE, Thaventhiran T, Bergstrom E, Gardener Z, Ascough S, Chiu C, Docherty AB, Hunt D, Crow YJ, Solomon T, Taylor GP, Turtle L, Harrison EM, Dunning J, Semple MG, Baillie JK, Openshaw PJ | display-authors = 6 | title = Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19 | journal = Science Immunology | volume = 6 | issue = 57 | pages = eabg9873 | date = March 2021 | pmid = 33692097 | doi = 10.1126/sciimmunol.abg9873 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Zhao Y, Kilian C, Turner JE, Bosurgi L, Roedl K, Bartsch P, Gnirck AC, Cortesi F, Schultheiß C, Hellmig M, Enk LU, Hausmann F, Borchers A, Wong MN, Paust HJ, Siracusa F, Scheibel N, Herrmann M, Rosati E, Bacher P, Kylies D, Jarczak D, Lütgehetmann M, Pfefferle S, Steurer S, Zur-Wiesch JS, Puelles VG, Sperhake JP, Addo MM, Lohse AW, Binder M, Huber S, Huber TB, Kluge S, Bonn S, Panzer U, Gagliani N, Krebs CF | display-authors = 6 | title = Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients | journal = Science Immunology | volume = 6 | issue = 56 | date = February 2021 | pmid = 33622974 | doi = 10.1126/sciimmunol.abf6692|pmc=8128299 | doi-access = free }}</ref> |
GM-CSF stimulates [[monocyte]]s and [[macrophage]]s to produce pro-inflammatory cytokines, including [[CCL17]].<ref name="pmid33150139" /> Elevated GM-CSF has been shown to contribute to inflammation in [[inflammatory arthritis]], [[osteoarthritis]], [[colitis]] [[asthma]], [[obesity]], and [[Coronavirus disease 2019|COVID-19]].<ref name="pmid33150139" /><ref name=Thwaites>{{cite journal | vauthors = Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, Liew F, Russell CD, Moore SC, Fairfield C, Carter E, Abrams S, Short CE, Thaventhiran T, Bergstrom E, Gardener Z, Ascough S, Chiu C, Docherty AB, Hunt D, Crow YJ, Solomon T, Taylor GP, Turtle L, Harrison EM, Dunning J, Semple MG, Baillie JK, Openshaw PJ | display-authors = 6 | title = Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19 | journal = Science Immunology | volume = 6 | issue = 57 | pages = eabg9873 | date = March 2021 | pmid = 33692097 | doi = 10.1126/sciimmunol.abg9873|pmc=8128298 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Zhao Y, Kilian C, Turner JE, Bosurgi L, Roedl K, Bartsch P, Gnirck AC, Cortesi F, Schultheiß C, Hellmig M, Enk LU, Hausmann F, Borchers A, Wong MN, Paust HJ, Siracusa F, Scheibel N, Herrmann M, Rosati E, Bacher P, Kylies D, Jarczak D, Lütgehetmann M, Pfefferle S, Steurer S, Zur-Wiesch JS, Puelles VG, Sperhake JP, Addo MM, Lohse AW, Binder M, Huber S, Huber TB, Kluge S, Bonn S, Panzer U, Gagliani N, Krebs CF | display-authors = 6 | title = Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients | journal = Science Immunology | volume = 6 | issue = 56 | date = February 2021 | pmid = 33622974 | doi = 10.1126/sciimmunol.abf6692|pmc=8128299 | doi-access = free }}</ref> |
||
==Clinical trials== |
==Clinical trials== |
||
[[Monoclonal antibody|Monoclonal antibodies]] against GM-CSF are being used as treatment in clinical trials against [[rheumatoid arthritis]], [[ankylosing spondylitis]], and COVID-19.<ref name="pmid33150139">{{cite journal | vauthors = Lee KM, Achuthan AA, Hamilton JA | title = GM-CSF: A Promising Target in Inflammation and Autoimmunity | journal = ImmunoTargets and Therapy | volume = 9 | pages = 225–240 | year = 2020 | pmid = 33150139 | pmc = 7605919 | doi = 10.2147/ITT.S262566 }}</ref> |
[[Monoclonal antibody|Monoclonal antibodies]] against GM-CSF are being used as treatment in clinical trials against [[rheumatoid arthritis]], [[ankylosing spondylitis]], and COVID-19.<ref name="pmid33150139">{{cite journal | vauthors = Lee KM, Achuthan AA, Hamilton JA | title = GM-CSF: A Promising Target in Inflammation and Autoimmunity | journal = ImmunoTargets and Therapy | volume = 9 | pages = 225–240 | year = 2020 | pmid = 33150139 | pmc = 7605919 | doi = 10.2147/ITT.S262566 | doi-access = free }}</ref> |
||
== See also == |
== See also == |
||
* [[CFU-GM]] |
* [[CFU-GM]] |
||
| ⚫ | |||
* [[Granulocyte-macrophage colony-stimulating factor receptor]] |
* [[Granulocyte-macrophage colony-stimulating factor receptor]] |
||
* [[Lenzilumab]] |
|||
| ⚫ | |||
* [[Pegfilgrastim]] (Neulasta, a [[PEGylated]] form [[filgrastim]]) |
* [[Pegfilgrastim]] (Neulasta, a [[PEGylated]] form [[filgrastim]]) |
||
Latest revision as of 11:59, 30 August 2023
| Granulocyte-macrophage colony-stimulating factor | |||||||||
|---|---|---|---|---|---|---|---|---|---|
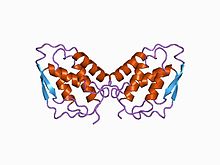 three-dimensional structure of recombinant human granulocyte-macrophage colony-stimulating factor (rhGM_CSF) | |||||||||
| Identifiers | |||||||||
| Symbol | GM_CSF | ||||||||
| Pfam | PF01109 | ||||||||
| Pfam clan | CL0053 | ||||||||
| InterPro | IPR000773 | ||||||||
| PROSITE | PDOC00584 | ||||||||
| SCOP2 | 2gmf / SCOPe / SUPFAM | ||||||||
| |||||||||
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| Chemical and physical data | |
| Formula | C639H1006N168O196S8 |
| Molar mass | 14434.54 g·mol−1 |
| | |
Granulocyte-macrophage colony-stimulating factor (GM-CSF), also known as colony-stimulating factor 2 (CSF2), is a monomeric glycoprotein secreted by macrophages, T cells, mast cells, natural killer cells, endothelial cells and fibroblasts that functions as a cytokine. The pharmaceutical analogs of naturally occurring GM-CSF are called sargramostim and molgramostim.
Unlike granulocyte colony-stimulating factor, which specifically promotes neutrophil proliferation and maturation, GM-CSF affects more cell types, especially macrophages and eosinophils.[5]
Function[edit]
GM-CSF is a monomeric glycoprotein that functions as a cytokine—it is a white blood cell growth factor.[6] GM-CSF stimulates stem cells to produce granulocytes (neutrophils, eosinophils, and basophils) and monocytes. Monocytes exit the circulation and migrate into tissue, whereupon they mature into macrophages and dendritic cells. Thus, it is part of the immune/inflammatory cascade, by which activation of a small number of macrophages can rapidly lead to an increase in their numbers, a process crucial for fighting infection.[citation needed]
GM-CSF also has some effects on mature cells of the immune system. These include, for example, enhancing neutrophil migration and causing an alteration of the receptors expressed on the cells surface.[7]
GM-CSF signals via signal transducer and activator of transcription, STAT5.[8] In macrophages, it has also been shown to signal via STAT3. The cytokine activates macrophages to inhibit fungal survival. It induces deprivation in intracellular free zinc and increases production of reactive oxygen species that culminate in fungal zinc starvation and toxicity.[9] Thus, GM-CSF facilitates development of the immune system and promotes defense against infections.[citation needed]
GM-CSF also plays a role in embryonic development by functioning as an embryokine produced by reproductive tract.[10]
Genetics[edit]
The human gene has been localized in close proximity to the interleukin 3 gene within a T helper type 2-associated cytokine gene cluster at chromosome region 5q31, which is known to be associated with interstitial deletions in the 5q- syndrome and acute myelogenous leukemia. GM-CSF and IL-3 are separated by an insulator element and thus independently regulated.[11] Other genes in the cluster include those encoding interleukins 4, 5, and 13.[12]
Glycosylation[edit]
Human granulocyte-macrophage colony-stimulating factor is glycosylated in its mature form.[citation needed]
History[edit]
GM-CSF was first cloned in 1985, and soon afterwards three potential drug products were being made using recombinant DNA technology: molgramostim was made in Escherichia coli and is not glycosylated, sargramostim was made in yeast, has a leucine instead of proline at position 23 and is somewhat glycosylated, and regramostim was made in Chinese hamster ovary cells (CHO) and has more glycosylation than sargramostim. The amount of glycosylation affects how the body interacts with the drug and how the drug interacts with the body.[13]
At that time, Genetics Institute, Inc. was working on molgramostim,[14] Immunex was working on sargramostim (Leukine),[15] and Sandoz was working on regramostim.[16]
Molgramostim was eventually co-developed and co-marketed by Novartis and Schering-Plough under the trade name Leucomax for use in helping white blood cell levels recover following chemotherapy, and in 2002 Novartis sold its rights to Schering-Plough.[17][18]
Sargramostim was approved by the US FDA in 1991 to accelerate white blood cell recovery following autologous bone marrow transplantation under the trade name Leukine, and passed through several hands, ending up with Genzyme,[19] which was subsequently acquired by Sanofi. Leukine is now owned by Partner Therapeutics (PTx).
Imlygic was approved by the US FDA in October 2015,[20] and in December 2015 by the EMA, as an oncolytic virotherapy, commercialized by Amgen Inc. This oncolytic herpes virus, named Talimogene laherparepvec, has been genetically engineered to express human GM-CSF using the tumor cells machinery.[21]
Clinical significance[edit]
GM-CSF is found in high levels in joints with rheumatoid arthritis and blocking GM-CSF as a biological target may reduce the inflammation or damage. Some drugs (e.g. otilimab) are being developed to block GM-CSF.[22] In critically ill patients GM-CSF has been trialled as a therapy for the immunosuppression of critical illness, and has shown promise restoring monocyte[23] and neutrophil[24] function, although the impact on patient outcomes is currently unclear and awaits larger studies.
GM-CSF stimulates monocytes and macrophages to produce pro-inflammatory cytokines, including CCL17.[25] Elevated GM-CSF has been shown to contribute to inflammation in inflammatory arthritis, osteoarthritis, colitis asthma, obesity, and COVID-19.[25][26][27]
Clinical trials[edit]
Monoclonal antibodies against GM-CSF are being used as treatment in clinical trials against rheumatoid arthritis, ankylosing spondylitis, and COVID-19.[25]
See also[edit]
- CFU-GM
- Filgrastim (Neupogen, a granulocyte colony-stimulating factor (G-CSF) analog)
- Granulocyte-macrophage colony-stimulating factor receptor
- Lenzilumab
- Pegfilgrastim (Neulasta, a PEGylated form filgrastim)
References[edit]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000164400 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000018916 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Root RK, Dale DC (March 1999). "Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: comparisons and potential for use in the treatment of infections in nonneutropenic patients". The Journal of Infectious Diseases. 179 (Suppl 2): S342-52. doi:10.1086/513857. PMID 10081506.
- ^ Francisco-Cruz A, Aguilar-Santelises M, Ramos-Espinosa O, Mata-Espinosa D, Marquina-Castillo B, Barrios-Payan J, Hernandez-Pando R (January 2014). "Granulocyte-macrophage colony-stimulating factor: not just another haematopoietic growth factor". Medical Oncology. 31 (1): 774. doi:10.1007/s12032-013-0774-6. PMID 24264600. S2CID 24452892.
- ^ Gasson JC (March 1991). "Molecular physiology of granulocyte-macrophage colony-stimulating factor". Blood. 77 (6): 1131–45. doi:10.1182/blood.V77.6.1131.1131. PMID 2001448.
- ^ Voehringer D (October 2012). "Basophil modulation by cytokine instruction". European Journal of Immunology. 42 (10): 2544–50. doi:10.1002/eji.201142318. PMID 23042651. S2CID 23972211.
- ^ Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS (October 2013). "Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival". Immunity. 39 (4): 697–710. doi:10.1016/j.immuni.2013.09.006. PMC 3841917. PMID 24138881.
- ^ Hansen PJ, Dobbs KB, Denicol AC (September 2014). "Programming of the preimplantation embryo by the embryokine colony stimulating factor 2". Animal Reproduction Science. 149 (1–2): 59–66. doi:10.1016/j.anireprosci.2014.05.017. PMID 24954585.
- ^ Bowers SR, Mirabella F, Calero-Nieto FJ, Valeaux S, Hadjur S, Baxter EW, et al. (April 2009). "A conserved insulator that recruits CTCF and cohesin exists between the closely related but divergently regulated interleukin-3 and granulocyte-macrophage colony-stimulating factor genes". Molecular and Cellular Biology. 29 (7): 1682–93. doi:10.1128/MCB.01411-08. PMC 2655614. PMID 19158269.
- ^ "Entrez Gene: CSF2 colony stimulating factor 2 (granulocyte-macrophage)".
- ^ Armitage JO (December 1998). "Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor" (PDF). Blood. 92 (12): 4491–508. doi:10.1182/blood.V92.12.4491. PMID 9845514.
- ^ "Molgramostim". AdisInsight. Retrieved 3 April 2018.
- ^ Staff (May 2008). "Back to the Future: Original Liquid Leukine® Coming Soon" (PDF). Oncology Business Review. Archived from the original (PDF) on 2016-08-25. Retrieved 2016-08-29.
- ^ Hussein AM, Ross M, Vredenburgh J, Meisenberg B, Hars V, Gilbert C, et al. (November 1995). "Effects of granulocyte-macrophage colony stimulating factor produced in Chinese hamster ovary cells (regramostim), Escherichia coli (molgramostim) and yeast (sargramostim) on priming peripheral blood progenitor cells for use with autologous bone marrow after high-dose chemotherapy". European Journal of Haematology. 55 (5): 348–56. doi:10.1111/j.1600-0609.1995.tb00713.x. PMID 7493686. S2CID 25424116.
- ^ "Press release: Novartis Oncology sharpens focus on key growth drivers". Novartis via SEC Edgar. 30 October 2002.
- ^ "Scientific Conclusions and Grounds for Amendment of the Summary of Product Characteristics Presented by the EMEA" (PDF). EMA CPMP. 27 June 2000.
- ^ "Bayer Healthcare Pharmaceuticals Plant, Snohomish County, Washington State". pharmaceutical-technology.com. Retrieved 12 November 2011.
- ^ U.S. Food & Drug Administration. "IMLYGIC (talimogene laherparepvec)". fda.gov. Retrieved 17 December 2019.
- ^ Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. (September 2015). "Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma". Journal of Clinical Oncology. 33 (25): 2780–8. doi:10.1200/JCO.2014.58.3377. PMID 26014293.
- ^ Deiß A, Brecht I, Haarmann A, Buttmann M (March 2013). "Treating multiple sclerosis with monoclonal antibodies: a 2013 update". Expert Review of Neurotherapeutics. 13 (3): 313–35. doi:10.1586/ern.13.17. PMID 23448220. S2CID 169334.
- ^ Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, et al. (October 2009). "Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial". American Journal of Respiratory and Critical Care Medicine. 180 (7): 640–8. doi:10.1164/rccm.200903-0363OC. PMID 19590022.
- ^ Pinder EM, Rostron AJ, Hellyer TP, Ruchaud-Sparagano MH, Scott J, Macfarlane JG, et al. (October 2018). "Randomised controlled trial of GM-CSF in critically ill patients with impaired neutrophil phagocytosis". Thorax. 73 (10): 918–925. doi:10.1136/thoraxjnl-2017-211323. PMC 6166597. PMID 30064991.
- ^ a b c Lee KM, Achuthan AA, Hamilton JA (2020). "GM-CSF: A Promising Target in Inflammation and Autoimmunity". ImmunoTargets and Therapy. 9: 225–240. doi:10.2147/ITT.S262566. PMC 7605919. PMID 33150139.
- ^ Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, Liew F, Russell CD, Moore SC, et al. (March 2021). "Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19". Science Immunology. 6 (57): eabg9873. doi:10.1126/sciimmunol.abg9873. PMC 8128298. PMID 33692097.
- ^ Zhao Y, Kilian C, Turner JE, Bosurgi L, Roedl K, Bartsch P, et al. (February 2021). "Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients". Science Immunology. 6 (56). doi:10.1126/sciimmunol.abf6692. PMC 8128299. PMID 33622974.
External links[edit]
- Official gentaur web site
- Official Leukine web site
- Granulocyte-Macrophage+Colony-Stimulating+Factor at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P04141 (Granulocyte-macrophage colony-stimulating factor) at the PDBe-KB.