Midostaurin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
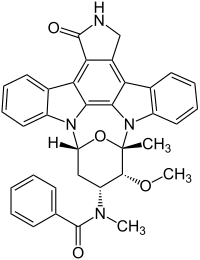
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Midostaurin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 35 H 30 N 4 O 4 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 570.646 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
235-260 ° C |
|||||||||||||||
| solubility | ||||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Midostaurin (trade name Rydapt ) is a tyrosine kinase inhibitor that has been studied in the treatment of acute myeloid leukemia , myelodysplastic syndrome, and advanced systemic mastocytosis . It is a semi-synthetic derivative of staurosporine , an alkaloid from the bacterium Streptomyces staurosporeus . The active ingredient has been approved in the USA since 2017. In Europe, the European Medicines Agency for FLT3-positive AML and three forms of systemic mastocytosis received marketing authorization in September 2017.
literature
- FDA: FDA information
Individual evidence
- ↑ a b c Data sheet Midostaurin hydrate, ≥98% (HPLC), solid from Sigma-Aldrich , accessed on November 4, 2017 ( PDF ).
- ↑ a b Datasheet Midostaurin at AlfaAesar, accessed on November 4, 2017 ( PDF )(JavaScript required) .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Pharmaceutical newspaper online: Midostaurin: A new multitarget kinase inhibitor: Pharmaceutical newspaper online: Midostaurin: A new multitarget kinase inhibitor , accessed on November 4, 2017.
- ^ Hillard Lazarus, Molly Miller: Midostaurin: an emerging treatment for acute myeloid leukemia patients. In: Journal of Blood Medicine. , P. 73, doi : 10.2147 / JBM.S100283 .
- ^ Gordon W. Gribble: Heterocyclic Scaffolds II: Reactions and Applications of Indoles . Springer Science & Business Media, 2010, ISBN 978-3-642-15732-5 , p. 18 ( limited preview in Google Book search).
- ↑ Approval: Midostaurin is now an option for AML and mastocytosis: Approval: Midostaurin is now an option for AML and mastocytosis , accessed on September 1, 2019.