Dantron: Difference between revisions
Content deleted Content added
m Quick-adding category "quinones" (using HotCat) |
m Adding Chemspider ID |
||
| Line 3: | Line 3: | ||
| image = Dantron.svg |
| image = Dantron.svg |
||
| CAS_number = 117-10-2 |
| CAS_number = 117-10-2 |
||
| ChemSpiderID = 2845 |
|||
| ATC_prefix = A06 |
| ATC_prefix = A06 |
||
| ATC_suffix = AB03 |
| ATC_suffix = AB03 |
||
Revision as of 04:15, 10 October 2008
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, rectal (enema) |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.003.794 |
| Chemical and physical data | |
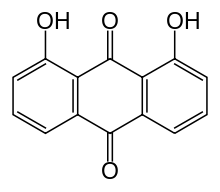
| Formula | C14H8O4 |
| Molar mass | 240.211 g/mol g·mol−1 |
- "Dantron" is also a trade name of ondansetron, an unrelated drug, in South Africa.
Dantron (also known as chrysazin) is an anthraquinone derivative, 1,8-dihydroxyanthraquinone, used in some countries as a stimulant laxative. In the USA it is considered to be a carcinogen,[1] and is therefore not used. In the UK it is considered a possible carcinogen and so its licence is restricted to patients who already have a diagnosis of terminal cancer (i.e. it is mainly used in palliative care to counteract the constipating effects of opioids)
It has the notable side-effect of causing red-coloured urine.
Danthron was the British Approved Name, but it has now been changed to "dantron" in harmony with the recommended International Nonproprietary Name (rINN).
References
- ^ Danthron substance profile at the National Toxicology Program website
