1,1,2,2-tetrafluoroethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
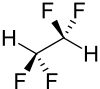
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,1,2,2-tetrafluoroethane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 2 F 4 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 102.03 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| Melting point |
−89 ° C |
|||||||||||||||
| boiling point |
−19.9 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Global warming potential |
1337 (based on 100 years) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1,1,2,2-Tetrafluoroethane is a chemical compound of fluorine from the group of fluorocarbons .
Extraction and presentation
1,1,2,2-Tetrafluoroethane can be obtained by hydrogenolysis of Cryofluoran .
use
In contrast to its isomer 1,1,1,2-tetrafluoroethane, 1,1,2,2-tetrafluoroethane is rarely used as a coolant because of its lower vapor pressure . It is used to foam polystyrene .
Individual evidence
- ^ A b David R. Lide: CRC Handbook of Chemistry and Physics, 85th Edition . CRC Press, 2004, ISBN 978-0-8493-0485-9 , pp. 6–145 ( limited preview in Google Book search).
- ↑ a b SynQuest Labs: 1,1,2,2-Tetrafluoroethane | SynQuest Labs, Inc. , accessed July 25, 2017.
- ↑ G. Myhre, D. Shindell et al .: Climate Change 2013: The Physical Science Basis . Working Group I contribution to the IPCC Fifth Assessment Report. Ed .: Intergovernmental Panel on Climate Change . 2013, Chapter 8: Anthropogenic and Natural Radiative Forcing, pp. 24-39; Table 8.SM.16 ( PDF ).
- ^ DD Eley, Herman Pines, Paul B. Weisz: Advances in Catalysis . Academic Press, 1993, ISBN 978-0-08-056543-9 , pp. 340 ( limited preview in Google Book search).
- ^ RE Banks, BE Smart, JC Tatlow: Organofluorine Chemistry Principles and Commercial Applications . Springer Science & Business Media, 2013, ISBN 978-1-4899-1202-2 , pp. 626 ( limited preview in Google Book search).
- ↑ United Nations: UNEP 2006 Report of the Rigid and Flexible Foams Technical Options Committee . United Nations Environment Program Ozone Secretariat, 2007, ISBN 978-92-807-2826-2 , pp. 24 ( limited preview in Google Book search).

