1,2-dioxetanedione
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | 1,2-dioxetanedione | |||||||||
| other names |
1,2-dioxetane-3,4-dione |
|||||||||
| Molecular formula | C 2 O 4 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 88.02 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
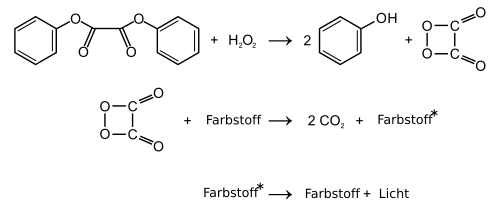
1,2-Dioxetanedione has been proposed as a reactive intermediate in the chemiluminescence reaction of aryl oxalates with hydrogen peroxide ( peroxyoxalate chemiluminescence ). However, the existence of the heterocyclic compound appears to be uncertain. As a chemical compound , 1,2-dioxetanedione would belong to the class of dioxetanes and organic peroxides , especially the peroxylacetones. The four-ring heterocycle can also be understood as a cyclic oxide of carbon or as a dimer of carbon dioxide.
It has been postulated that 1,2-dioxetanedione breaks down into two molecules of carbon dioxide in an exergonic reaction. The energy is to be transferred to dye molecules ( fluorescent dyes ), whereby the phenomenon of chemiluminescence occurs.
Further intermediate stages were discussed for energy transfer. The detection of 1,2-dioxetanedione in the gas phase by mass spectroscopic methods has been denied. [ 4] In solution, however, the compound could be identified by 13 C-NMR spectroscopic examination of the reaction of 13 C-labeled oxalyl chloride with hydrogen peroxide.
use
In glow sticks are used as starting materials for generating the postulated 1,2-Dioxetandions derivatives of oxalic acid used. Examples are bis (2,4,6-trichlorophenyl) oxalate (TCPO) or bis (2,3-dinitrophenyl) oxalate (DNPO). However, the starting material most frequently used commercially today is bis (2,4,5-trichlorophenyl-6-carbopentoxyphenyl) oxalate (CPPO). All of these starting materials are acylating agents ("reactive esters") and, when hydrogen peroxide is added, should react to form two equivalents of the (substituted) phenol and 1,2-dioxetanedione.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ MM Rauhut, BG Roberts, AM Semael, J. Am. Chem. Soc. , 88, 3604 (1966).
- ^ MM Rauhut, Chemiluminescence from Concerted Peroxide Decomposition Reaction, Accounts of Chemical Research , 2, 80-87 (1969).
- ↑ Waldemar Adam and Faris Yany, 1,2-Dioxetanes and α-Peroxylactones in: Alfred Hassner: Chemistry of Heterocyclic Compounds: Small Ring Heterocycles, Part 3: Oxiranes, Arene Oxides, Oxaziridines, Dioxetanes, Thietanes, Thietes, Thiazetes, and Others , Volume 42, pp. 351-420, especially p. 370, John Wiley & Sons 1985, ISBN 978-0-47018-720-3 .
- ↑ Herbert Brandl, Chemiluminescence, in: Dieter Wöhrle, Michael W. Tausch, Wolf-Dieter Stohrer, Photochemistry: Concepts, Methods, Experiments , Chapter 6, pp. 244–249, Wiley-VCH, Weinheim u. a. O., 1998, ISBN 3-527-29545-3 .
- ↑ Herman F. Cordes, Herbert P. Richter, Carl A. Heller: Mass spectrometric evidence for the existence of 1,2-dioxetanedione (carbon dioxide dimer). Chemiluminescent intermediate . In: J. Am. Chem. Soc. . 91, No. 25, 1969, p. 7209. doi : 10.1021 / ja01053a065 .
- ↑ J. Stauff, W. Jaeschke, G. Schlögl: Chemiluminescence of "Dioxetandione". In: Journal of Nature Research B . 27, 1972, p. 1434 ( online ).
- ↑ JJ DeCorpo, A. Baranowski, MV McDowell, FE Saalfeld, Formation of carbon dioxide dimer in chemiluminescent reactions, J. Am. Chem. Soc. , 1972, 94 (8), 2879-2880. DOI: 10.1021 / ja00763a067 .
- ↑ Richard Bos, Neil W. Barnett, Gail A. Dyson, Kieran F. Lim, Richard A. Russell, Simon P. Watson, Studies on the mechanism of the peroxyoxalate chemiluminescence reaction: Part 1. Confirmation of 1,2-dioxetanedione as an intermediate using 13 C nuclear magnetic resonance spectroscopy, Analytica Chimica Acta 502, 2141-147 (2004), DOI: 10.1016 / j.aca.2003.10.014 .
- ↑ Sarah A. Tonkin, Richard Bos, Gail A. Dyson, Kieran F. Lim, Richard A. Russell, Simon P. Watson, Christopher M. Hindson, Neil W. Barnett, Studies on the mechanism of the peroxyoxalate chemiluminescence reaction: Part 2. Further identification of intermediates using 2D EXSY 13C nuclear magnetic resonance spectroscopy, Analytica Chimica Acta , 614, 2173-181 (2008), DOI: 10.1016 / j.aca.2008.03.009 .