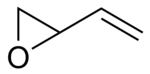
3,4-epoxy-1-butene
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | 3,4-epoxy-1-butene | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 4 H 6 O | |||||||||
| Brief description |
colorless liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 70.09 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
0.87 g cm −3 (25 ° C) |
|||||||||
| boiling point |
|
|||||||||
| solubility |
soluble in ethanol, diethyl ether, benzene and other organic solvents |
|||||||||
| Refractive index |
1.417 (20 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
3,4-Epoxy-1-butene is a chemical compound from the oxirane group and is the epoxide of 1,3-butadiene .
Extraction and presentation
3,4-Epoxy-1-butene can be obtained by epoxidizing 1,3-butadiene.
properties
3,4-epoxy-1-butene is a colorless clear liquid.
use
3,4-Epoxy-1-butene is used as an intermediate in the production of other chemical compounds (such as 2,5-dihydrofuran ) and as a comonomer with propylene oxide for polyethers .
Individual evidence
- ↑ a b c d e f g h data sheet 3,4-epoxy-1-butene, 98% from Sigma-Aldrich , accessed on September 6, 2015 ( PDF ).
- ↑ a b Data sheet (R) -2-Vinyloxirane, ≥95.0% (sum of enantiomers, GC) from Sigma-Aldrich , accessed on September 6, 2015 ( PDF ).
- ↑ Data sheet (S) -2-Vinyloxirane, technical, ≥90% (sum of enantiomers, GC) from Sigma-Aldrich , accessed on September 6, 2015 ( PDF ).
- ↑ a b Entry on 3,4-epoxy-1-butenes in the Hazardous Substances Data Bank , accessed on September 6, 2015.
- ↑ T. Otto, P. Pfeifer, S. Pitter, B. Powietzka: Organic synthesis in a microreactor - heterogeneously catalyzed epoxidation of 1,3-butadiene. In: Chemical Engineer Technology. 81, 2009, p. 349, doi : 10.1002 / cite.200800151 .

