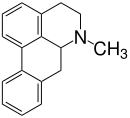
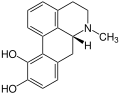
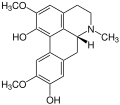
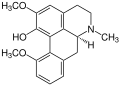
Aporphine alkaloids
Aporphine alkaloids are naturally occurring chemical compounds from the group of alkaloids . After the benzylisoquinoline alkaloids, they represent the second largest group of isoquinoline alkaloids .
To date, 85 aporphine alkaloids have been isolated from plants of 15 families; the best known representative is the apomorphine . The aporphine alkaloids are of particular interest because of their proximity to morphine .
Occurrence


The aporphine alkaloids are most commonly found in plants.
For example, isoboldine occurs in the plants Beilschmiedia, Nandina ( Nandina domestica , or sky bamboo ), horn poppy ( Glaucium ) and other plants. As the name suggests, the glaucine was the first to be detected in the horn poppy ( Glaucium ) and the name of the alkaloids is usually derived from the plants in which they were first detected.
Corydin as another representative of the aporphine alkaloids is contained in the lark spurs ( Corydalis ), heart flowers ( Dicentra ) and also in the horn poppy ( Glaucium ) .
Representative
The aporphine alkaloids differ in their different substituents and in their position on the basic structure. Furthermore, their stereochemistry is partly different, most often they are ( R ) -configured. B. glaucine , bulbocapnine and isothebaine ( S ) -configured.
biosynthesis
Reticulin 1 is oxidized in the first step, resulting in a mesomeric-stabilized diradical with the boundary structures 2a and 2b . Cyclization creates a fourth six-membered ring: Corytuberin 3, which then dehydrates to Bulbocapnin 4 . Schematic representation:
In the laboratory, aporphine alkaloids such as isoboldine or isothebaine can also be synthesized from reticulin through oxidative phenol coupling using potassium hexacyanoferrate or iron (III) chloride . Proaporphins can also easily be converted into aporphine alkaloids.
chemistry
The aporphine alkaloids are of particular interest because of their proximity to morphine and the benzylisoquinoline alkaloids. For example, as the name suggests, morphine can be used to produce apomorphine. This can be done by adding an acid under the influence of heat.
The proaporphine alkaloids and the aporphine alkaloids share a skeletal isomerism.
The aporphine alkaloids usually have a stereocenter .
The ( R ) -configured glaucin can be produced synthetically from ( S ) -glaucin.
use
Aporphine alkaloids are predominantly of pharmacological importance as many of them act on organisms. The pharmacological spectrum of action is very broad and the modes of action on the different species are very different:
In rabbits, isothebaine has been shown to increase intestinal muscle tone . In rats it caused stimulation of the uterine muscles and an anti-inflammatory effect. In mice, on the other hand, motor disorders and reduced pain perception can occur.
Glaucin is said to reduce blood pressure and has a breathing inhibiting effect in cats. In addition, it should suppress the urge to cough in a similar way to codeine , but it should have a longer duration of action than codeine. The derivative of the glaucine dehydroglaucine has an antibacterial effect.
Corydin acts as a sedative, lowers blood pressure and blocks nerve impulses .
Bulbocapnine is said to block the effects of apomorphine and amphetamine . It acts on the central nervous system and can cause catalepsy in mice . However, it should also have a calming and pain relieving effect.
Apomorphine has an antihypertensive effect and is also a powerful emetic . It is mainly used as a remedy for Parkinson's disease because of its stimulating effect on dopamine receptors .
In African folk medicine, the plant Cassytha ( Cassytha filiformis ) is considered a drug against cancer . One study showed that the plant contains many aporphine alkaloids and the three main alkaloids Actinodaphnin , Cassythin and Dicentrin in vitro affect cancer cells.
literature
- M. Hesse, HO Bernhard: Alkaloids: except indole, triterpene and steroid alkaloids . Verlag Chemie, Weinheim 1975, ISBN 3-527-25620-2 .
- Geoffrey A. Cordell: Introduction to Alkaloids: A Biogenetic Approach . John Wiley & Sons, Canada 1981, ISBN 0-471-03478-9 .
- P. Nuhn, L. Wessjohann: Natural product chemistry: Microbial, vegetable and animal natural products. S. Hirzel Verlag, Stuttgart 2006, ISBN 3-7776-1363-0 .
Individual evidence
- ^ KW Bentley, HME Cardwell: The Morphine-Thebaine group of alkaloids. Part V. The absolute stereochemistry of the morphine, benzylisoquinoline, aporphine, and tetrahydroberberine alkaloids. In: Journal of the Chemical Society. Oxford 1955, pp. 3252-3260, doi: 10.1039 / JR9550003252 .
- ↑ G. Blaschke: Mechanism of the diphenyl linkage in the biosynthesis of aporphine alkaloids. 3rd communication: Investigation into the biosynthesis of alkaloids. In: Archives of Pharmacy. 303 (4), 1970, pp. 358-363, doi: 10.1002 / ardp.19703030411 .
- ^ Geoffrey A. Cordell: Introduction to Alkaloids: A Biogenetic Approach . John Wiley & Sons, Canada 1981, ISBN 0-471-03478-9 , pp. 406-408.
- ↑ S. Hoet, C. Stevigny, S. Block, F. Opperdoes, P. Colson, B. Baldeyrou, A. Lansiaux, C. Bailly, J. Quetin-Leclercq: Alkaloids from Cassytha filiformis and related aporphines: antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases. In: Planta Med. 70 (5), Thieme Verlag, Stuttgart 2004, pp. 407-413, doi: 10.1055 / s-2004-818967 .