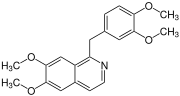
Benzylisoquinoline alkaloids
The benzyl (tetrahydro) isoquinoline alkaloids are natural substances of the isoquinoline alkaloid type, which are derived from benzylisoquinoline .
Occurrence
Benzylisoquinoline alkaloids are u. a. Formed in the plant families of the poppy family , annone family and laurel family . Well-known representatives are found primarily in poppy plants or the opium obtained from them, but also in Christopher's herbs . For example, the reticulin was isolated from the Netzannone .
Well-known representatives
More than 2500 biologically active derivatives of the benzylisoquinoline alkaloids are known. Due to their structure, the substances can be divided into numerous subgroups: the aporphines, the phthalidisoquinoline alkaloids , the morphinans , the protoberberine alkaloids and the pavins.
Papaverine is one of the known individual substances in this group of substances . Further representatives are u. a. Reticulin and laudanosine .
properties
Papaverine has vasodilating and muscle relaxing properties. Laudanosine acts as a tetanic poison .
biosynthesis
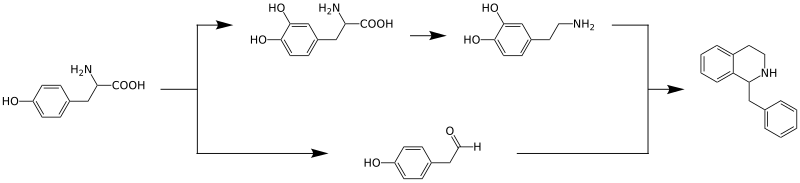
The biosynthesis of the benzylisoquinoline alkaloids has been studied intensively. It begins with the amino acid tyrosine , which is converted into dopamine through hydroxylation and decarboxylation and into 4-hydroxyphenylacetaldehyde through oxidative deamination . These two compounds are linked in a condensation reaction catalyzed by the enzyme norcoclaurine synthase to form the benzylisoquinoline backbone.
The benzylisoquinoline from this reaction can have different substituents ; reticulin is an important intermediate.
Individual evidence
- ↑ a b c Entry on benzyl (tetrahydro) isoquinoline alkaloids. In: Römpp Online . Georg Thieme Verlag, accessed on May 22, 2020.
- ^ Hermann Hager: Hager's Handbook of Pharmaceutical Practice: Volume 2: Methods , 1133 pages, Verlag Springer (1991), ISBN 978-3-540-52459-5 , p. 35 ( limited preview in the Google book search).
- ↑ a b Eberhard Breitmaier: Alkaloids . Springer Fachmedien, Wiesbaden 1997, ISBN 978-3-519-03542-8 , pp. 62 .
- ↑ Bettina Ruff: Chemical and biochemical methods for the stereoselective synthesis of complex natural substances , 199 pages, Verlag Logos Berlin (2012), ISBN 978-3-8325-3121-8 , p. 8 ( limited preview in the Google book search).
- ↑ a b Jennifer M. Finefield, David H. Sherman, Martin Kreitman, Robert M. Williams: Enantiomeric Natural Products: Occurrence and Biogenesis. In: Angewandte Chemie. 124, 2012, pp. 4886-4920, doi: 10.1002 / anie.201107204 .
- ^ A b Yang-Chang Wu: New Research and Development on the Formosan Annonaceous Plants . In: Studies in Natural Products Chemistry . tape 33 , 2007, p. 957-1023 , doi : 10.1016 / s1572-5995 (06) 80044-x (English).